Chapter: 11th Chemistry : UNIT 9 : Solutions
Elevation of boiling point
Elevation of boiling point
Boiling point is an important physical property of a liquid. The boiling point of a liquid is the temperature at which its vapour pressure becomes equal to the atmospheric pressure (1 atm). When a nonvolatile solute is added to a pure solvent at its boiling point, the vapour pressure of the solution is lowered below 1 atm. To bring the vapour pressure again to 1 atm, the temperature of the solution has to be increased. As a result,the solution boils at a higher temperature (Tb) than the boiling point of the pure solvent (Tb┬░). This increase in the boiling point is known as elevation of boiling point. A plot of vapour pressure versus temperature for water and an aqueous solution is given below
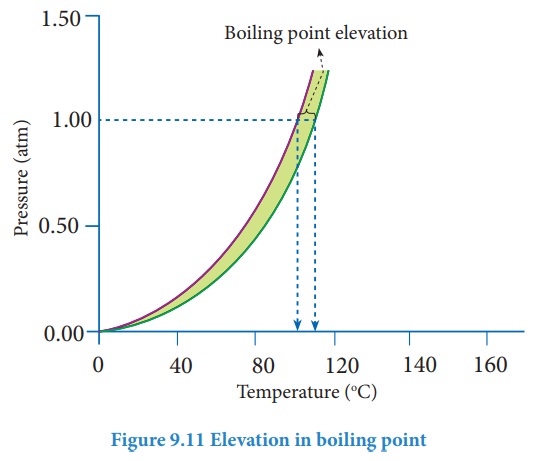
The vapour pressure of the solution increases with increase in temperature as shown in the above figure. The variation of vapour pressure with respect to temperature for pure water is given by the violet coloured curve. At 100 Ōü░C the vapour pressure of water is equal to 1 atm. Hence the boiling point of water is 100 Ōü░C (Tb┬░). When a solute is added to water, the vapour pressure of the resultant solution is lowered. The variation of vapour pressure with respect to temperature for the solution is given by green curve. From the graph, it evident the vapour pressure of the solution is equal to 1 atm pressure at the temperature Tb which is greater than Tb┬░. The difference between these two temperatures (Tb-Tb┬░) gives the elevation of boiling point.
The elevation of boiling point (ΔTb)= Tb - Tb°
The elevation of boiling point is directly proportional to the concentration of the solute particles.
ΔTb α m (9.23)
m is the concentration of solution expressed in molality.
ΔTb = Kb m (9.24)
Where
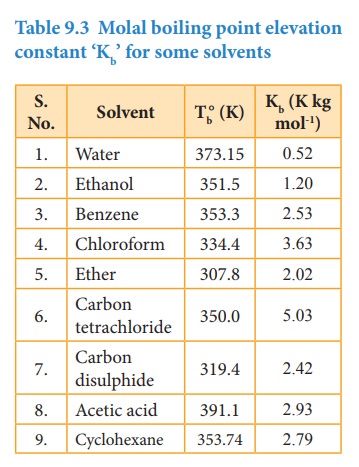
Kb = molal boiling point elevation constant or Ebullioscopic constant.
If m=1, then ΔTb=Kb;
Hence, the Kb is equal to the elevation in boiling point for 1 molal solution. Kb is calculated by the following expression
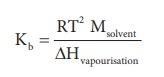
Problem:
0.75 g of an unknown substance is dissolved in 200 g water. If the elevation of boiling point is 0.15 K and molal elevation constant is 7.5 K Kg mol-1 then, calculate the molar mass of unknown substance
ΔTb = Kb m
= Kb x W2 x 1000 / M2 x W1
M2 = Kb x W2 x 1000 / ΔTb x W1
= 7.5 x 0.75 x 1000 / 0.15 x 200
= 187.5 g mol-1
Related Topics