Chemistry - Expressing concentration of solutions | 11th Chemistry : UNIT 9 : Solutions
Chapter: 11th Chemistry : UNIT 9 : Solutions
Expressing concentration of solutions
Expressing
concentration of solutions
In
our life we have come across many solutions of varying strengths or
concentrations such as mouthwash, antiseptic solutions, household disinfectants
etc... Have you ever noticed the concentration of the ingredients present in
those solutions? For example, chlorhexidine mouthwash solution contains 0.2 %
(w/v) chlorhexidine gluconate; The concentration of the commercially available
hydrogen peroxide is 3% (w/v). Similarly, other terms such as ppm (TDS of
water), molar and normal (laboratory reagents) are used to express the
concentration of the solution. The concentration of a solution gives the amount
of solute present in a given quantity of solvent. As we have seen, there are
different ways of expressing the concentration of a solutions. Let us learn the
different concentration terms and to prepare a solution of a specific
concentration.
Table 9.2 Different concentration units and their illustrations
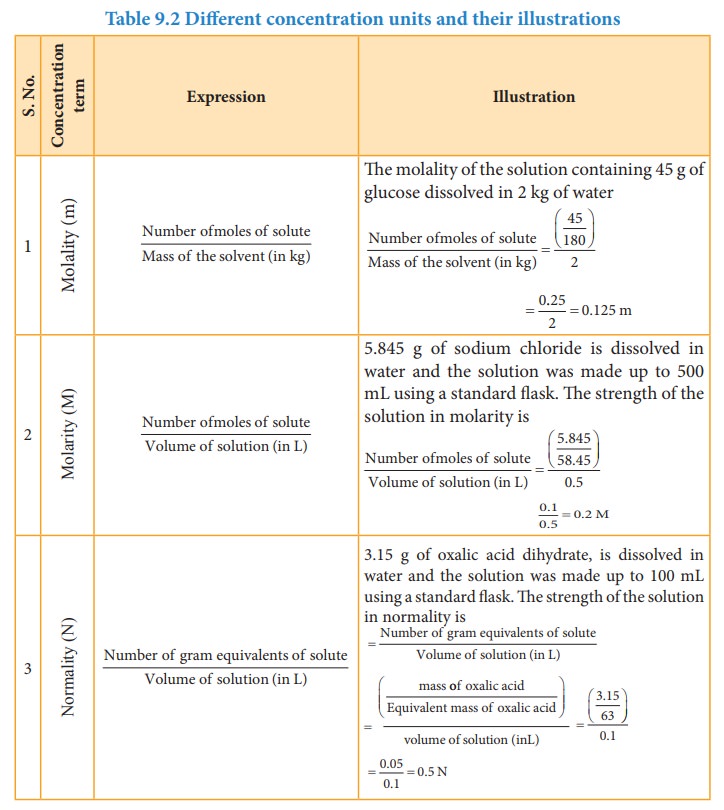
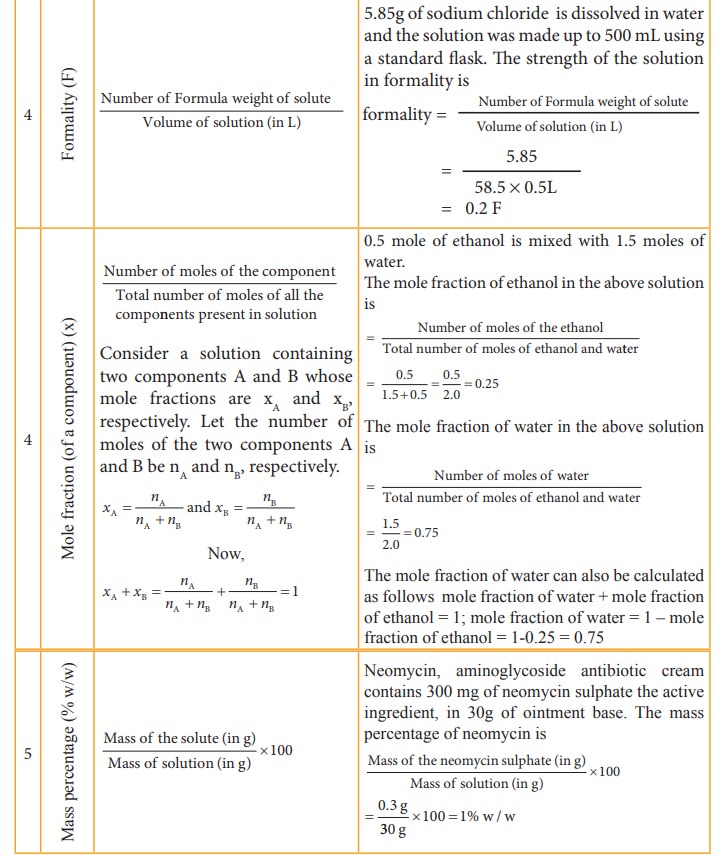
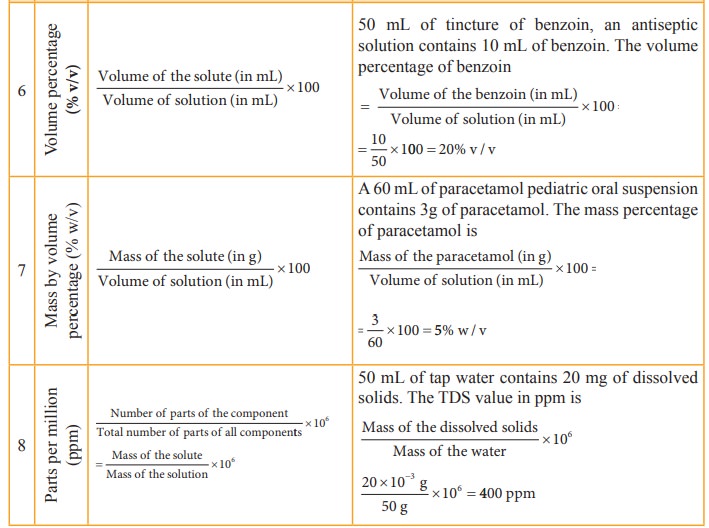
The
concentration of a solution is expressed in different units. The choice of unit
depends on the type of measurement applied. For example,in complexometric
titrations involving EDTA, the reaction between EDTA and the metal ions takes
place in the 1:1 mole ratio and hence molar solutions are used in this
titrations. In the redox and neutralisation titrations we use normality. The
mole fraction is used to calculate the partial pressure of gases and the vapour
pressure of solutions. The percentage units are used to express the active
ingredients present in therapeutics, and the ppm is used to express the
quantity of solutes present in small amounts in solutions.
Standard solutions and working standards
A
standard solution or a stock solution is a solution whose concentration is
accurately known. A standard solution of required concentration can be prepared
by dissolving a required amount of a solute, in a suitable amount of solvent.
Its done by transforming a known amount of a solute to a standard flask of
definite volume. A small amount of water is added to the flask and shaken well
to dissolve the salt.. Then water is added to the flask to bring the solution
level to the mark indicated at the top end of the flask. The flask is stoppered
and shaken well to make concentration uniform.
At
the time of experiment, the solution with required concentration is prepared by
diluting the stock solution. This diluted solution is usually called working
standard. A known volume of stock solution is transferred to a new container
and brought to the calculated volume. The necessary volumes of the stock
solution and final volume can be calculated using the following expression.
Cs
Vs = Cw Vw (9.1)
Where
the Cs &Vs are concentration and the volume of the
stock solution and Cw & Vw are concentration and the
volume of the working standard, respectively.
![]()
![]()
Advantages of using standard solutions:
1.
The error due to weighing the solute can be minimised by using concentrated
stock solution that requires large quantity of solute.
2.
We can prepare working standards of different concentrations by diluting the
stock solution, which is more efficient since consistency is maintained.
3.
Some of the concentrated solutions are more stable and are less likely to
support microbial growth than working standards used in the experiments.
Example Problem
1.
What volume of 4M HCl and 2M HCl should be mixed to get 500 mL of 2.5 M HCl?
Let
the volume of 4M HCl required to prepare 500 mL of 2.5 MHCl = x mL
Therefore,
the required volume of 2M HCl = (500 - x) mL
We
know from the equation (9.1)
C1V1+
C2V2 = C3V3
(4x)+2(500-x) = 2.5
√ó 500
4x+1000-2x = 1250
2x = 1250 -
1000
x = 250/2
= 125 mL
Hence, volume of 4M HCl required = 125 mL
Volume
of 2M HCl required = (500 - 125) mL= 375 mL
Related Topics