Chapter: 11th Chemistry : UNIT 9 : Solutions
Factors responsible for deviation of solution from RaoultŌĆÖs law
Factors responsible for deviation from RaoultŌĆÖs law
The deviation of solution from ideal behavior is attributed to the following factors.
i) Solute-solvent interactions
For an ideal solution, the interaction between the solvent molecules (A-A),the solute molecules (B-B) and between the solvent & solute molecules (A-B) are expected to be similar. If these interactions are dissimilar, then there will be a deviation from ideal behavior.
ii) Dissociation of solute
When a solute present in a solution dissociates to give its constituent ions, the resultant ions interact strongly with the solvent and cause deviation from RaoultŌĆÖs law.
For example, a solution of potassium chloride in water deviates from ideal behavior because the solute dissociates to give K+ and ClŌĆō ion which form strong ion-dipole interaction with water molecules.
KCl (s) + H2O (l) ŌåÆ K+ (aq)+ ClŌĆō (aq)
iii)Association of solute
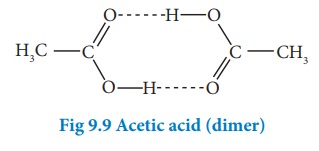
Association of solute molecules can also cause deviation from ideal behaviour. For example, in solution, acetic acid exists as a dimer by forming intermolecular hydrogen bonds, and hence deviates from RaoultŌĆÖs law.
iv) Temperature
An increase in temperature of the solution increases the average kinetic energy of the molecules present in the solution which causes decrease in the attractive force between them. As result, the solution deviates from ideal behaviour.
v) Pressure
At high pressure the molecules tend to stay close to each other and therefore there will be an increase in their intermolecular attraction. Thus, a solution deviates from RaoultŌĆÖs law at high pressure.
vi) Concentration
If a solution is sufficiently dilute there is no pronounced solvent-solute interaction because the number of solute molecules are very low compared to the solvent. When the concentration is increased by adding solute, the solvent-solute interaction becomes significant. This causes deviation from the RaoultŌĆÖs law.
Related Topics