Solutions | Chemistry - Henry's law | 11th Chemistry : UNIT 9 : Solutions
Chapter: 11th Chemistry : UNIT 9 : Solutions
Henry's law
Henry's
law
William
Henry investigated the relationship between pressure and solubility of a
gaseous solute in a particular solvent. According to him, ŌĆ£the partial pressure
of the gas in vapour phase (vapour pressure of the solute) is directly
proportional to the mole fraction(x) of the gaseous solute in the solution at
low concentrationsŌĆØ. This statement is known as HenryŌĆÖs law.
HenryŌĆÖs
law can be expressed as,
psolute ╬▒ xsolute in solution (9.1)
psolute = KHxsolute in solution (9.2)
Here,
psolute represents the partial pressure of the gas in vapour state
which is commonly called as vapour pressure. xsolute in solution
represents the mole fraction of solute in the solution. KH is a
empirical constant with the dimensions of pressure. The value of ŌĆśKHŌĆÖ
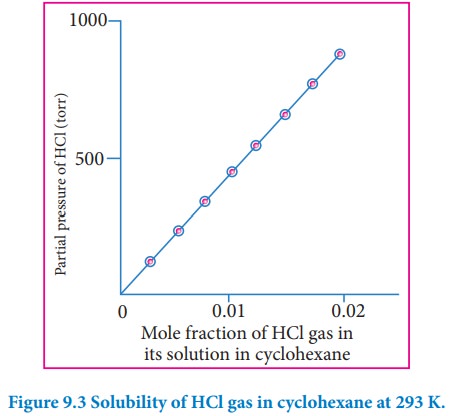
depends on the nature of the gaseous solute and solvent. The above equation is
a straight-line in the form of y=mx. The plot partial pressure of the gas
against its mole fraction in a solution will give a straight line as shown in
fig 9.3. The slope of the straight line gives the value of KH.
Limitations of HenryŌĆÖs law
i.
HenryŌĆÖs law is applicable at moderate temperature and pressure only.
ii.
Only the less soluble gases obeys HenryŌĆÖs law
iii.
The gases reacting with the solvent do not obey HenryŌĆÖs law. For example,
ammonia or HCl reacts with water and hence does not obey this law.
NH3+ H2O Ōćå
NH4+ + OHŌĆō
iv.
The gases obeying HenryŌĆÖs law should not associate or dissociate while
dissolving in the solvent.
Example Problem 2:
0.24
g of a gas dissolves in 1 L of water at 1.5 atm pressure. Calculate the amount
of dissolved gas when the pressure is raised to 6.0 atm at constant
temperature.
psolute
= KHxsolute in solution
At
pressure 1.5 atm,
p1
= KH x1------------(1)
At
pressure 6.0 atm,
p2
= KH x2------------(2)
Dividing
equation (1) by (2)
From
equation p1/p2 =
x1/x2
1.5/6.0
= 0.24/x2
Therefore
x2 = 0.24 x 6.0/1.5 = 0.96 g/L
Why the carbonated drinks are stored in a pressurized container?
We
all know that the carbonated beverages contain carbon dioxide dissolved in
them. To dissolve the carbon dioxide in these drinks, the CO2 gas is
bubbled through them under high pressure. These containers are sealed to
maintain the pressure. When we open these containers at atmospheric pressure,
the pressure of the CO2 drops to the atmospheric level and hence
bubbles of CO2 rapidly escape from the solution and show
effervescence. The burst of bubbles is even more noticeable, if the soda bottle
is in warm condition.
Related Topics