Multiple choice questions - Choose the best answer: Chemistry: Solutions | 11th Chemistry : UNIT 9 : Solutions
Chapter: 11th Chemistry : UNIT 9 : Solutions
Choose the best answer: Chemistry: Solutions
Chemistry: Solutions
Choose the best answer
1. The molality of a solution containing 1.8g of glucose dissolved in 250g of water is
a) 0.2 M
b) 0.01 M
c) 0.02 M
d) 0.04 M
Solution:
molality = number of moles of solute / weight of solvent (in kg)
= (1.8/180) / 0.25 = 0.01/0.25 = 0.04M
2. Which of the following concentration terms is / are independent of temperature
a) molality
b) molarity
c) mole fraction
d) (a) and (c)
Solution:
option (d) is correct. Molality and mole fraction are independent of temperature.
3. Stomach acid, a dilute solution of HCl can be neutralised by reaction with Aluminium hydroxide
Al (OH)3 + 3HCl (aq) ŌåÆ AlCl3 + 3 H2O
How many millilitres of 0.1 M Al(OH)3 solution are needed to neutralise 21 mL of 0.1 M HCl ?
a) 14 mL
b) 7 mL
c) 21 mL
d) none of these
Solution:
M1 ├Ś V1 = M2 ├Ś V2 [ŌłĄ 0.1 M
Al(OH)3 gives 3 ├Ś 0.1 = 0.3 M OHŌĆō ions]
0.3 ├Ś V1 = 0.1 ├Ś 21
V1 = 01x21 / 0.3 = 7ml
4. The partial pressure of nitrogen in air is 0.76 atm and its Henry's law constant is 7.6 ├Ś 104 atm at 300K. What is the molefraction of nitrogen gas in the solution obtained when air is bubbled through water at 300K ?
a) 1 ├Ś 10ŌĆō4
b) 1 ├Ś 10ŌĆō6
c) 2 ├Ś 10ŌĆō5
d) 1 ├Ś 10ŌĆō5
Solution:
PN2= 0.76 atm
KH = 7.6 ├Ś 104
x = ?
PN2 = KH . x
0.76 = 7.6 ├Ś 104 ├Ś x
so x = 0.76 / [7.6 x104] = 1x10-5
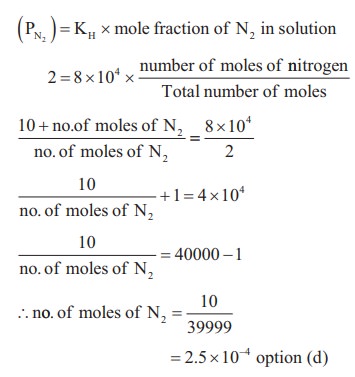
5. The Henry's law constant for the solubility of Nitrogen gas in water at 350 K is 8 ├Ś 104 atm. The mole fraction of nitrogen in air is 0.5. The number of moles of Nitrogen from air dissolved in 10 moles of water at 350K and 4 atm pressure is
a) 4 ├Ś 10ŌĆō4
b) 4 ├Ś 104
c) 2 ├Ś 10ŌĆō2
d) 2.5 ├Ś 10ŌĆō4
Solution:
KH = 8 ├Ś 104
(xN2)in air = 0.5
Total pressure = 4 atm
pressure Partial pressure of nitrogen = mole fraction ├Ś total pressure = 0.5 ├Ś 4 = 2
6. Which one of the following is incorrect for ideal solution ?
a) ŌłåHmix = 0
b) ŌłåUmix = 0
c) ŌłåP = Pobserved ŌĆō PCalculated by raoults law = 0
d) ŌłåGmix = 0
Solution:
for an ideal solution,
╬öSmix ŌēĀ 0 ; Hence ╬öGmix ŌēĀ 0
Ōł┤ incorrect is ╬öGmix = 0
7. Which one of the following gases has the lowest value of Henry's law constant ?
a) N2
b) He
c) CO2
d) H2
Solution:
Carbon dioxide ; most stable gas and has lowest value of Henrys Law constant.
8. P1 and P2 are the vapour pressures of pure liquid components, 1 and 2 respectively of an ideal binary solution if x1 represents the mole fraction of component 1, the total pressure of the solution formed by 1 and 2 will be
a) P1 + x1 (P2 ŌĆō P1)
b) P2 ŌĆō x1 (P2 + P1)
c) P1 ŌĆō x2 (P1 ŌĆō P2)
d) P1 + x2 (P1 ŌĆō P2)
Solution:
Ptotal = P1 + P2
= P1x1 + P2x2 (x1 + x2 = 1)
= P1 (1 ŌĆō x2) + P2x2 (x1 = 1 ŌĆō x2)
= P1 ŌĆō P1x2 + P2x2
= P1 ŌĆō x2 (P1 ŌĆō P2)
9. Osometic pressure (p) of a solution is given by the relation
a) ŽĆ = nRT
b) ŽĆV = nRT
c) ŽĆRT = n
d) none of these
Solution:
ŽĆ = CRT
ŽĆ = n/V . RT
ŽĆ V = nRT
10. Which one of the following binary liquid mixtures exhibits positive deviation from Raoults law ?
a) Acetone + chloroform
b) Water + nitric acid
c) HCl + water
d) ethanol + water
Solution:
Ethanol and water
11. The Henry's law constants for two gases A and B are x and y respectively. The ratio of mole fractions of A to B is 0.2. The ratio of mole fraction of B and A dissolved in water will be
a) 2x/y
b) y/0.2x
c) 0.2x/y
d) 5x/y
Solution:
Given, (KH)A = x
(KH)B = y
xA/xB = 0.2
(xA/xB)in solution = ?
PA = x (xA) in solution ŌĆō (1)
PB = y (xB) in solution ŌĆō (2)
(xA/xB)in solution = (PB/PA) . (x/y) = (xB/xA) . (x/y) = (1/0.2) . (x/y) = 5x/y
12. At 100oC the vapour pressure of a solution containing 6.5g a solute in 100g water is 732mm. If Kb = 0.52, the boiling point of this solution will be
a) 102oC
b) 100oC
c) 101oC
d) 100.52oC
Solution:
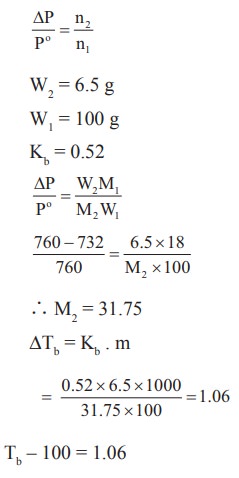
Tb ŌĆō 100 = 1.06
Tb = 100 + 1.06
= 101.06 Ōēł 101o C
13. According to Raoults law, the relative lowering of vapour pressure for a solution is equal to
a) mole fraction of solvent
b) mole fraction of solute
c) number of moles of solute
d) number of moles of solvent
Solution:
ŌłåP/P┬║= x2 (mole fraction of the solute)
14. At same temperature, which pair of the following solutions are isotonic ?
a) 0.2 M BaCl2 and 0.2M urea
b) 0.1 M glucose and 0.2 M urea
c) 0.1 M NaCl and 0.1 M K2SO4
d) 0.1 M Ba (NO3)2 and 0.1 M Na2 SO4
Solution:
0.1 ├Ś 3 ion [Ba2+, 2NO3ŌĆō]
= 0.1 ├Ś 3 ion [2 Na+, SO4ŌĆō]
15. The empirical formula of a non-electrolyte(X) is CH2O. A solution containing six gram of X exerts the same osmotic pressure as that of 0.025M glucose solution at the same temperature. The molecular formula of X is
a) C2H4O2
b) C8H16O8
c) C4H8O4
d) CH2O
Solution:
(ŽĆ1)non electrolyte = (ŽĆ2)glucose
C1RT = C2RT
[CH2O => 12+2+16=30]
W1/M1 = W1/M2
6 / n(30) = 0.025
n = 8
Ōł┤ molecular formula = C8H16O8
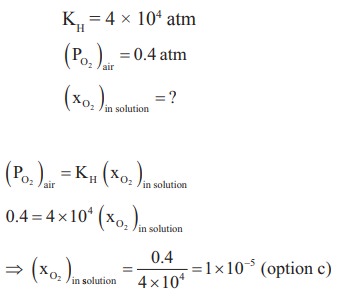
16. The KH for the solution of oxygen dissolved in water is 4 ├Ś 104 atm at a given temperature. If the partial pressure of oxygen in air is 0.4 atm, the mole fraction of oxygen in solution is
a) 4.6 ├Ś 103
b) 1.6 ├Ś 104
c) 1 ├Ś 10ŌĆō5
d) 1 ├Ś 105
Solution:
17. Normality of 1.25 M sulphuric acid is
a) 1.25 N
b) 3.75 N
c) 2.5 N
d) 2.25 N
Solution:
Normality of H2SO4 = (no.of replacable H+)├ŚM
= 2 ├Ś 1.25
= 2.5 N
18. Two liquids X and Y on mixing gives a warm solution. The solution is
a) ideal
b) non-ideal and shows positive deviation from Raoults law
c) ideal and shows negative deviation from Raoults Law
d) non-ideal and shows negative deviation from Raoults Law
Solution:
ΔHmix is negative and show negative deviation from Raoults law.
19. The relative lowering of vapour pressure of a sugar solution in water is 3.5 ├Ś 10ŌĆō3. The mole fraction of water in that solution is
a) 0.0035
b) 0.35
c) 0.0035 / 18
d) 0.9965
Solution:
ŌłåP / P┬║ = Xsugar
3.5 ├Ś 10ŌĆō3 = Xsugar
Xsugar + XH2o = 1
XH2o = 1 ŌĆō 0.0035 = 0.9965
20. The mass of a non-voltaile solute (molar mass 80 g molŌĆō1) which should be dissolved in 92g of toluene to reduce its vapour pressure to 90%
a) 10g
b) 20g
c) 9.2 g
d) 8.89g
Solution:
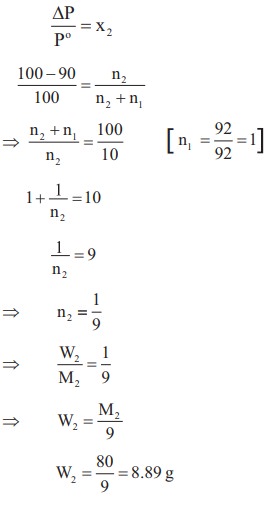
21. For a solution, the plot of osmotic pressure (p) verses the concentration (c in mol LŌĆō1) gives a straight line with slope 310R where 'R' is the gas constant. The temperature at which osmotic pressure measured is
a) 310 ├Ś 0.082 K
b) 310 ┬║ C
c) 37oC
d) 310/0.082 K
Solution:
ŽĆ = CRT
y = x (m)
m = RT
310 R = RT
Ōł┤ T = 310 K
= 37┬║ C
22. 200ml of an aqueous solution of a protein contains 1.26g of protein. At 300K, the osmotic pressure of this solution is found to be 2.52 ├Ś 10ŌĆō3 bar. The molar mass of protein will be (R = 0.083 L bar molŌĆō1KŌĆō1)
a) 62.22 Kg molŌĆō1
b) 12444g molŌĆō1
c) 300g molŌĆō1
d) none of these
Solution:
ŽĆ = CRT
ŽĆ = [ W/MV ] . RT
M = WRT / ŽĆ V
= [1.26 x 0.083x300] / [2.52x10-3x0.2]
= 62.22 Kg mol-1
23. The Van't Hoff factor (i) for a dilute aqueous solution of the strong elecrolyte barium hydroxide is
a) 0
b) 1
c) 2
d) 3
Solution:
Ba (OH)2 dissociates to form
Ba2+ and 2OH ion
╬▒=(i-1) / (n-1)
i= ╬▒(n-1)+1
n=i=3 (for Ba(OH)2), ╬▒ =1)
24. What is the molality of a 10% W/W aqueous sodium hydroxide solution ?
a) 2.778
b) 2.5
c) 10
d) 0.4
Solution:
10% W/W aqueous NaOH solution means that 10 g of sodium hydroxide in 100g solution
Molality = no. of moles of solute / weight of solvent (in kg)
= (10/40) / 0.1 = 0.25/0.1 = 2.5M
25. The correct equation for the degree of an associating solute, 'n' molecules of which undergoes association in solution, is
a) ╬▒ = n(i ŌłÆ1)/ (n ŌłÆ1)
b) ╬▒2 = n(1 ŌłÆ i) / n ŌłÆ1
c) ╬▒ = n(i ŌłÆ1)/(1-i)
d) ╬▒ = n(1 ŌłÆ n) / n(1-i)
Solution:
╬▒=(1-i)n/(n-1) or n(i-1)/(1-n)
26. Which of the following aqueous solutions has the highest boiling point ?
a) 0.1 M KNO3
b) 0.1 M Na3PO4
c) 0.1 M BaCl2
d) 0.1 M K2SO4
Solution:
Elevation of boiling point is more in the case of Na3PO4 (no. of ions 4 ; 3 Na+, PO43ŌĆō)
27. The freezing point depression constant for water is 1.86o K Kgmol-1. If 5g Na2SO4 is dissolved in 45g water, the depression in freezing point is 3.64oC. The Vant Hoff factor for Na2SO4 is
a) 2.50
b) 2.63
c) 3.64
d) 5.50
Solution:
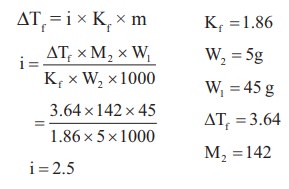
28. Equimolal aqueous solutions of NaCl and KCl are prepared. If the freezing point of NaCl is ŌĆō2┬║C, the freezing point of KCl solution is expected to be
a) ŌĆō2oC
b) ŌĆō 4oC
c) ŌĆō 1oC
d) 0oC
Solution:
Equimolal aqueous solution of KCl also shows 2┬║C depression in freezing point.
29. Phenol dimerises in benzene having van't Hoff factor 0.54. What is the degree of association ?
a) 0.46
b) 92
c) 46
d) 0.92
Solution:
i = 0.54
╬▒=(1-i)n/(n-1)
=(1-0.54)2 / (2-1)
╬▒= 0.92
30. Assertion : An ideal solution obeys Raoults Law
Reason : In an ideal solution, solvent-solvent as well as solute-solute interactions are similar to solute-solvent interactions.
a) both assertion and reason are true and reason is the correct explanation of assertion
b) both assertion and reason are true but reason is not the correct explanation of assertion
c) assertion is true but reason is false
d) both assertion and reason are false
Solution:
both assertion and reason are correct and reason is the correct explanation of assertion.
Related Topics