Chapter: 11th Chemistry : UNIT 9 : Solutions
Vapour pressure of binary solution of liquid in liquids
Vapour pressure of binary solution of liquid in liquids
Now, let us consider a binary liquid solution formed by dissolving a liquid solute ŌĆśAŌĆÖ in a pure solvent ŌĆśBŌĆÖ in a closed vessel. Both the components A and B present in the solution would evaporate and an equilibrium will be established between the liquid and vapour phases of the components A and B.
The French chemist Raoult, proposed a quantitative relationship between the partial pressures and the mole fractions of two components A & B, which is known as RaoultŌĆÖs Law. This law states that ŌĆ£in the case of a solution of volatile liquids, the partial vapour pressure of each component (A & B) of the solution is directly proportional to its mole fractionŌĆØ.
According to RaoultŌĆÖs law,
pA╬▒ xA (9.3)
pA = k xA
when xA = 1, k = p┬░A
where p┬░A is the vapour pressure of pure component ŌĆśAŌĆÖ at the same temperature.
Therefore,
pA = p┬░AXA (9.4)
Similarly, for component ŌĆśBŌĆÖ
pB= p┬░B xB (9.5)
xA and xB are the mole fraction of the components A and B respectively.
According to DaltonŌĆÖs law of partial pressure the total pressure in a closed vessel will be equal to the sum of the partial pressures of the individual components.
Hence,
Ptotal = pA + pB (9.6)
Substituting the values of pA and pB from equations (9.4) and (9.5) in the above equation,
Ptotal = xAp┬░A + xBp┬░B (9.7)
We know that xA + xB = 1 or xA = 1 - xB
Therefore,
Ptotal = (1 - xB) p┬░A + xB p┬░B (9.8)
Ptotal= p┬░A + xB( p┬░B- p┬░A) (9.9)
The above equation is of the straight-line equation form y = mx+c. The plot of Ptotal versus xa will give a straight line with (pB┬░- pA┬░) as slope and pA┬░ as the y intercept.
Let us consider the liquid solution containing toluene (solute) in benzene (solvent).
The variation of vapour pressure of pure benzene and toluene with its mole fraction is given in the graph.
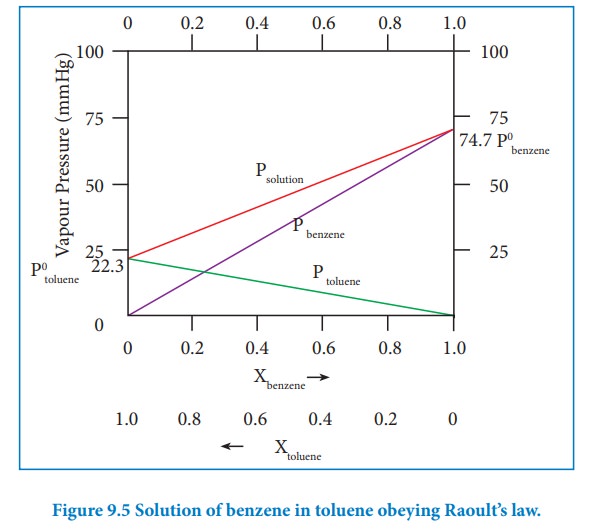
The vapour pressures of pure toluene and pure benzene are 22.3 and 74.7 mmHg, respectively. The above graph shows, the partial vapour pressure of the pure components increases linearly with the increase in the mole fraction of the respective components. The total pressure at any composition of the solute and solvent is given by the following straight line (represented as red line) equation.
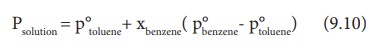
Related Topics