Physics - Classification of materials | 12th Physics : UNIT 10a : Semiconductor Electronics
Chapter: 12th Physics : UNIT 10a : Semiconductor Electronics
Classification of materials
Classification of materials
The classification of solids into insulators, metals, and semiconductors can be explained with the help of the energy band diagram.
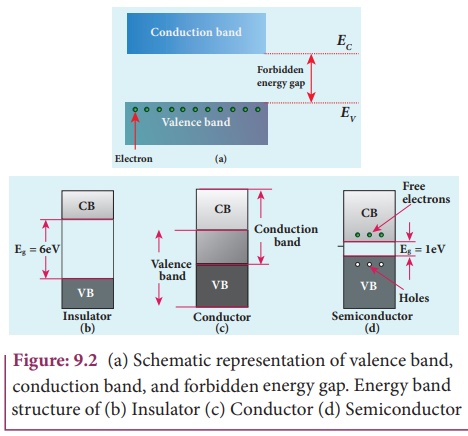
Insulators
The energy band structure of insulators is shown in Figure 9.2(b). The valence band and the conduction band are separated by a large energy gap. The forbidden energy gap is approximately 6 eV in insulators. The gap is very large that electrons from valence band cannot move into conduction band even on the application of strong external electric field or the increase in temperature. Therefore, the electrical conduction is not possible as the free electrons are almost nil and hence these materials are called insulators. Its resistivity is in the range of 1011–1019 Ωm.
Conductors
In condutors, the valence band and conduction band overlap as shown in Figure 9.2(c). Hence, electrons can move freely into the conduction band which results in a large number of free electrons in the conduction band. Therefore, conduction becomes possible even at low temperatures. The application of electric field provides sufficient energy to the electrons to drift in a particular direction to constitute a current. For condutors, the resistivity value lies between 10–2 and 10–8 Ωm.
Semiconductors
In semiconductors, there exists a narrow forbidden energy gap (Eg < 3eV ) between the valence band and the conduction band.
At a finite temperature, thermal agitations in the solid can break the covalent bond between the atoms (covalent bond is formed due to the sharing of electrons to attain stable electronic configuration). This releases some electrons from valence band to conduction band. Since free electrons are small in number, the conductivity of the semiconductors is not as high as that of the conductors. The resistivity value of semiconductors is from 10–5 to 106 Ωm
In semiconductors, electrons in the valence band are bound electrons which cannot move. Hence, they cannot contribute for conduction.
When the temperature is increased further, more number of electrons is promoted to the conduction band and increases the conduction. Thus, we can say that the electrical conduction increases with the increase in temperature. In other words, resistance decreases with increase in temperature. Hence, semiconductors are said to have negative temperature coefficient of resistance. The most important elemental semiconductor materials are Silicon (Si) and Germanium (Ge). The forbidden energy gaps for Si and Ge at at room temperature are 1.1 eV and 0.7 eV respectively.
Related Topics