Electro Chemistry | Chemistry - Variation of molar conductivity with concentration | 12th Chemistry : UNIT 9 : Electro Chemistry
Chapter: 12th Chemistry : UNIT 9 : Electro Chemistry
Variation of molar conductivity with concentration
Variation of molar conductivity with concentration
Friedrich Kohlraush studied the molar conductance of different
electrolytes at different concentrations. He observed that, increase of the
molar conductance of an electrolytic solution with the increase in the
dilution. One such experimental results is given in the following table for
better understanding.
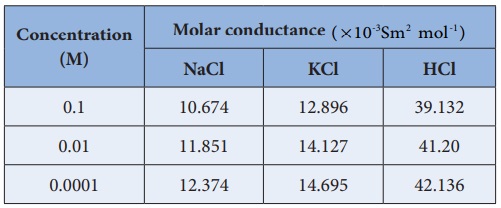
Based on the above such results, Kohlraush deduced the following
empirical relationship between the molar conductance (╬øm ) and
the concentration of the electrolyte (C).
╬ø m = ╬øm - k ŌłÜC .....(9.11)
The above equation represents a straight line of the form y = mx + c .
Hence, the plot of ╬øm Vs ŌłÜC gives a
straight line with a negative slope of ŌĆōk and the y intercept, ╬øm . Where ╬ø m is
called the limiting molar conductivity. i.e., the molar conductance approaches
a limiting value in very dilute solutions.
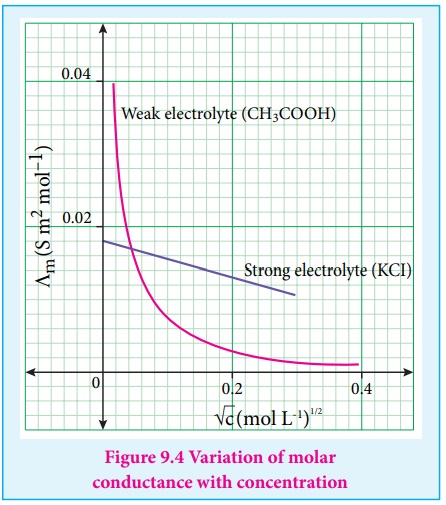
For strong electrolytes such as KCl, NaCl etc., the plot, ╬øm Vs ŌłÜC , gives a straight line as shown in the graph (9.4). It is also observed that the plot is not a linear one for weak electrolytes.
For a strong electrolyte, at high concentration, the number of constituent ions of the electrolyte in a given volume is high and hence the attractive force between the oppositely charged ions is also high. Moreover the ions also experience a viscous drag due to greater solvation. These factors attribute for the low molar conductivity at high concentration. When the dilution increases, the ions are far apart and the attractive forces decrease. At infinite dilution the ions are so far apart, the interaction between them becomes insignificant and hence, the molar conductivity increases and reaches a maximum value at infinite dilution.
For a weak electrolyte, at high concentration, the plot is almost
parallel to concentration axis with slight increase in conductivity as the
dilution increases. When the concentration approaches zero, there is a sudden
increase in the molar conductance and the curve is almost parallel to ╬øm axis.
This is due to the fact that the dissociation of the weak electrolyte increases
with the increase in dilution (Ostwald dilution law). ╬ø0m values
for strong electrolytes can be obtained by extrapolating the straight line, as
shown in figure (9.4). But the same procedure is not applicable for weak
electrolytes, as the plot is not a linear one, ╬ø0m values of the weak electrolytes can be determined
using Kohlraush's law.
Debye - Huckel and Onsager equation
We have learnt that at infinite dilution, the interaction between the
ions in the electrolyte solution is negligible. Except this condition,
electrostatic interaction between the ions alters the properties of the solution
from those expected from the free ŌĆō ions value. The influence of ion-ion
interactions on the conductivity of strong electrolytes was studied by Debye
and Huckel. They considered that each ion is surrounded by an ionic atmosphere
of opposite sign, and derived an expression relating the molar conductance of
strong electrolytes with the concentration by assuming complete dissociation.
Later, the equation was further developed by Onsager. For a uni ŌĆō univalent
electrolyte the Debye Huckel and Onsager equation is given below.
╬ø m = ╬ø0m ŌłÆ ( A + B ╬ø0m ) ŌłÜC .....(9.12)
Where A and B are the constants which depend only on the nature of the
solvent and temperature. The expression for A and B are
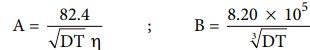
Here, D is the dielectric constant of the medium, ╬Ę the
viscosity of the medium and T the temperature in Kelvin.
Kohlraush's law
The limiting molar conductance ╬ø0m is the basis for kohlraush law. At infinite
dilution, the limiting molar conductivity of an electrolyte is equal to the sum
of the limiting molar conductivities of its constituent ions. i.e., the molar
conductivity is due to the independent migration of cations in one direction
and anions in the opposite direction.
For a uni ŌĆō univalent electrolyte such as NaCl, the Kohlraush's law is
expressed as
(╬ø0m )NaCl = ( ╬╗0m )Na+ + ( ╬╗0m )Cl-
In general, according to Kohlraush's law, the molar conductivity at
infinite dilution for a electrolyte represented by the formula Ax By
, is given below.
(╬ø0m)AxBy = x ( ╬╗0m
)Ay+ + y ( ╬╗0m )Bx- .....(9.13)
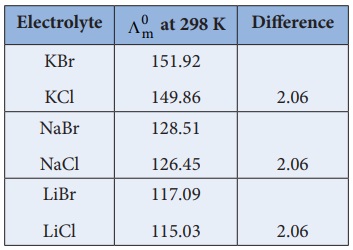
Kohlraush arrived the above mentioned relationship based on the
experimental observations such as the one as shown in the table. These result
show that at infinite dilution each constituent ion of the electrolyte makes a
definite contribution towards the molar conductance of the electrolyte
irrespective of nature of other ion with which it is associated
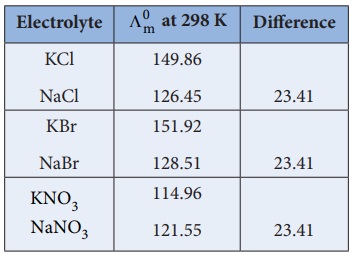
i.e.,
(╬ø┬║m )KCl ŌłÆ ( ╬ø┬║m)NaCl = 149.86 - 126.45
{( ╬╗┬║m )K+ + ( ╬╗┬║m )Cl- } ŌĆō {( ╬╗┬║m )Na+ + ( ╬╗┬║m)Cl- } = 23.41
( ╬╗┬║m ) K+ - ( ╬╗┬║m )Na+ = 23.41
Similarly, we can conclude that ( ╬╗┬║m )Br- ŌłÆ ( ╬╗┬║m )Cl- = 2.06
Applications of Kohlrausch's Law
1. Calculation of molar conductance at infinite dilution of a weak electrolyte.
It is impossible to determine the molar conductance at infinite dilution for weak electrolytes experimentally. However, the same can be calculated using KohlraushŌĆÖs Law.
For example, the molar conductance of CH3COOH, can be calculated using the experimentally determined molar conductivities of strong electrolytes HCl, NaCl and CH3COONa .
╬ø┬║CH3COONa = ╬╗┬║Na+ + ╬╗┬║CHCOO- .....(1)
╬ø┬║HCl = ╬╗┬║H+ + ╬╗┬║Cl- .....(2)
╬ø┬║NaCl = ╬╗┬║Na+ + ╬╗┬║Cl- .....(3)
Equation
(1) + Equation (2) ŌĆō Equation (3) gives,
╬ø┬║CH3COONa + ╬ø┬║HCl - ╬ø┬║NaCl = ╬╗oH+ + ╬╗oCH3COO-
= ╬ø┬║CH3COOH
2. Calculation of degree of dissociation of weak electrolytes
The degree of dissociation of weak electrolyte can be calculated from
the molar conductivity at a given concentration and the molar conductivity at
infinite dilution using the following expression

Calculation of dissociation constant using ╬øm values.
According to Ostwald dilution Law,

Substitute ╬▒ value in
the above expression ( 9.15)
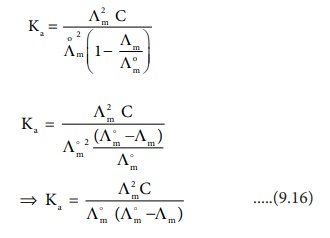
3. Calculation of solubility of sparingly soluble salts
Substances like AgCl, PbSO4 etc., are sparingly soluble in
water. The solubility product of such substances can be determined using
conductivity measurements.
Let us consider AgCl as an example
AgCl (s) Ōåö Ag+ + ClŌłÆ
Ksp = [Ag+ ][Cl- ]
Let the concentration of [Ag + ] be ŌĆśCŌĆÖ molLŌĆō1.
As per the stoichiometry, if [Ag+ ] = C, then [ClŌłÆ]also
equal to ŌĆśCŌĆÖ mol LŌłÆ1 .
Ksp =C.C
Ksp = C2
We know that the concentration (in mol dm-3) is related to
the molar and specific conductance by the following expressions
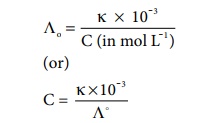
Substitute the concentration value in the relation Ksp = C2
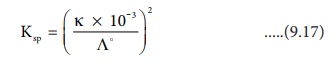
Related Topics