Electro Chemistry - Short Questions Answer | 12th Chemistry : UNIT 9 : Electro Chemistry
Chapter: 12th Chemistry : UNIT 9 : Electro Chemistry
Short Questions Answer
Chemistry : Electro Chemistry
Short Answer Questions
1. Define anode and cathode
i)
Anode: The electrode at which the oxidation
occurs is called the anode.
ii)
It is negative in a galvanic cell.
i)
Cathode: The electrode at which the
reduction occurs is called the cathode.
ii) It is positive in a galvanic cell.
2. Why does conductivity of a solution decrease on dilution of the solution
On
dilution of the electrolyte solution, the ions present in the unit dimension
was decreased and hence the conductivity of a solution also decreases.
3. State Kohlrausch Law. How is it useful to determine the molar conductivity of weak electrolyte at infinite dilution.
Kohlrausch Law:
At
infinite dilution, the limiting molar conducutivity of an electrolyte is equel
to the sum of the limiting molar conductivites of its constituent ions.
The
molar conductance at infinite dilution for weak electrolytes can be calculated
using KohlrauschŌĆÖs law.
Molar conductivity weak electrolyte
:
The
molar conductance of CH3COOH, can be calculated using the
experimentally determind molar conductivities of strong electrolytes HCl, NaCl
and CH3COONa.
╔ģoHCl3COONa
= ╬╗oNa + ╬╗oCH3COO- ŌĆ”ŌĆ”ŌĆ”ŌĆ”. (1)
╔ģoHCl
= ╬╗oH+ + ╬╗oCl- ---------------(2)
╔ģoNaCl
= ╬╗oNa+ + ╬╗oCl- ŌĆ”ŌĆ”ŌĆ”...(3)
Equation
(1) + Equation (2) ŌĆō Equation (3) gives,
(╔ģoCH3COONa)
+ (╔ģoHCl) ŌĆō (╔ģoNaCl) = ╬╗oH+
+ ╬╗oCH3COO-
=
╔ģoCH3COOH
4. Describe the electrolysis of molten NaCl using inert electrodes
The
products of electrolysis of molten NaCl is sodium metal and Cl2 gas.
Na
+ eŌłÆ ŌåÆ Na
The
anion ClŌłÆ oxidized at the anode.
Cl
ŌåÆ ┬Į Cl2 + eŌłÆ
5. State FaradayŌĆÖs Laws of electrolysis
First law:
The
mass of the substance (m) liberated at an electrode during electrolysis is
directly proportional to the quantity of charge (Q) passed through the cell.
i.e.,
m ╬▒ Q
m
╬▒ lt or m= Z lt
Second law:
When
the same quantity of charge is passed through the solutions of different
electrolytes, the amount of substances liberated at the respective electrodes
are directly proportional to their electrochemical equivalents.
m1
ŌłØ Z1
m2
ŌłØ Z2
m1/Z1
= m2/Z2
Z
= Electrochemical equivalent
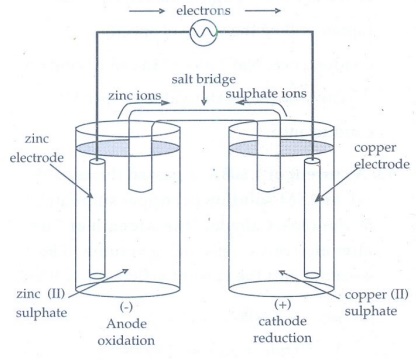
6. Describe the construction of Daniel cell. Write the cell reaction.
i)
Daniel cell or a galvanic cell is an example of electro chemical cell.
ii)
This overall reaction is made of the summation of oxidation half reaction and
reduction half reaction.
iii)
The oxidation half cell reaction occurring at the zinc electrode.
Zn(s)
ŌåÆ Zn2+(aq) + 2 eŌłÆ
iv)
The reduction half reaction occurring at the copper electrode. It receives the
electrons from the zinc electrode when connected externally, to produce
metallic copper according to the reaction as
Cu2+(aq)
+ 2eŌłÆ ŌåÆ Cu(s)
The
over all reaction taking place in the cell is the redox reaction gives as
Zn(s)
+ Cu2+(aq) ŌåÆ Zn2+(aq) + Cu(s)
v)
The decrease in the energy which appears as the heat energy when a zinc rod is
directly dipped into the ZnSO4 solution, is converted into
electrical energy. When the same reaction takes place indirectly in an electro
chemical cell.
vi)
When the cell is set up, electrons flow from zinc electrode through the wire to
the copper cathode.
vii)
As a result, zinc dissolves in the anode solution to form Zn2+ ions.
The Cu2+ ions in the cathode half cell pick up electrons and are
converted to Cu atoms on the cathode.
7. Why is anode in galvanic cell considered to be negative and cathode positive electrode?
ŌĆó Oxidation occurs at anode,
Electrons are liberated at anode and hence it is negative.
ŌĆó Reduction occurs at cathode. The
electrons are consumed at cathode and hence it is positive.
8. The conductivity of a 0.01M solution of a 1 :1 weak electrolyte at 298K is 1.5 ├Ś10-4 S cmŌłÆ1.
i) molar conductivity of the solution
ii) degree of dissociation and the dissociation constant of the weak electrolyte
Given that
╬╗┬║cation = 248.2 S cm2 molŌłÆ1
╬╗┬║cation = 51.8 S cm2 molŌłÆ1
Answer:
Given:
C
= 0.01M
╬╗┬░cation
= 248.2 S cm2 molŌłÆ1
K
= 1.5 ├Ś 10-4 S cmŌłÆ1
╬╗┬░anion
= 51.8 S cm2 molŌłÆ1
(i) molar conductivity
╔ģom
= [ k ├Ś10ŌłÆ3 / M ] molŌłÆ1m3
╔ģo
m= (1.5 ├Ś l0-4 ├Ś l0ŌłÆ3 ├Ś l02
) / ( 1 ├Ś10-2)
╔ģ┬░m
= 1.5 ├Ś 10ŌłÆ3 S m2 molŌłÆ1
(ii) Degree of dissociation
╬▒
= ╔ģom / ╔ģo╬▒
╔ģom
= ╬╗o+ + ╬╗o-
╔ģoŌł×
= ╬╗ocation + ╬╗oanion
=
(248.2 + 51.8) S cm2 molŌłÆ1
=
300 S cm2 molŌłÆ1
╔ģoŌł×
= 300 ├Ś 10-4 S m2 molŌłÆ1
╔ģom
= 1.5 ├Ś 10ŌłÆ3 Sm2 molŌłÆ1
╬▒ = ╔ģom
/ ╔ģoŌł× = (1.5 ├Ś 10ŌłÆ3 S m2 mo1ŌłÆ1)
/ (300 ├Ś 10-4 S m2
molŌłÆ1)
╬▒ = 0.05
Dissociation
constant
Ka
= ╬▒2C / 1-╬▒
=
(0.05)2(0.01) / 1-0.05
=
(25 ├Ś 10-4 ├Ś 10-2 ) / 95 ├Ś 10-2
= 0.26 ├Ś 10-4
=
2.6 ├Ś 10-5
Answer:
i)
Molar conductivity = 1.5 ├Ś 10ŌłÆ3 S m2 molŌłÆ1
ii)
Degree of dissociation = 0.05
Dissociation constant = 2.6 ├Ś 10-5
9. Which of 0.1M HCl and 0.1 M KCl do you expect to have greater ╬ø┬║m and why?
0.1
M HCl shows greater acidity
HCl
dissociated as H+ and ClŌłÆ
KCl
dissociated as K+ and ClŌłÆ
HCl
releases H+.
The
substance releases H+ is acid. Hence 0.1M HCl is more acidic than
0.1M KCl.
10. Arrange the following solutions in the decreasing order of specific conductance.
i) 0.01M KCl ii) 0.005M KCl iii) 0.1M KCl iv) 0.25 M KCl v) 0.5 M KCl
Answer:
i)
0.5 M KCl
ii)
0.25 M KCl
iii)
0.1 M KCl
iv)
0.01 M KCl
v)
0.005 M KCl
11. Why is AC current used instead of DC in measuring the electrolytic conductance?
When
DC current is applied through the conductivity cell, it will lead to the
electrolysis of the solution taken in the cell.
Hence
AC current is used for this measurement to prevent electrolysis.
12. 0.1M NaCl solution is placed in two different cells having cell constant 0.5 and 0.25cm-1 respectively. Which of the two will have greater value of specific conductance.
Solution:
K
= C ├Ś (Ōäō/A)
Specific
conductance is directly proportional to cell constant. Higher the cell constant
higher will be the value of specific conductance. NaCl placed in cell having
0.5 cmŌłÆ1 shows greater value of specific conductance.
13. A current of 1.608A is passed through 250 mL of 0.5M solution of copper sulphate for 50 minutes. Calculate the strength of Cu2+ after electrolysis assuming volume to be constant and the current efficiency is 100%.
Given: I =
1.608A;
t
= 50 min = 50 ├Ś 60 = 3000s
V = 500 mL; C = 0.5 M ; ╔│ = 100%
Solution:
The
number of Faradays of electricity passed through the CuSO4 solution
ŌćÆ Q = It
Q
= 1.608 ├Ś 3000
Q
= 4824 C
Ōł┤ Number of Faradays of
electricity
=
4824 C / 96500 C = 0.5F
Electrolysis
of CuSO4
Cu2+ (aq) + 2eŌłÆ ŌåÆ Cu(s)
The
above equation shows that 2F electricity will deposit 1 mole of Cu2+
to Cu.
Ōł┤ 0.5F electricity will deposit ( 1
mol / 2F ) ├Ś 0.5 F
= 0.025 mol
Initial
number of moles of Cu2+ in 250 ml of solution = ( 0.5 / 1000 mL ) ├Ś 250 mL
= 0.125 mol
Ōł┤ Concentration of Cu2+
= [ 0.1 mol / 250 mL ] ├Ś 1000 mL
= 0.4M
14. Can Fe3+ oxidises bromide to bromine under standard conditions?
Given: EFe 3+|Fe2+ = 0.771
EBr2|BrŌłÆ = 1.09V.
Answer:
Required
half cell reaction
2BrŌłÆ
ŌåÆ Br2 + 2eŌłÆ (E┬░ox) = ŌłÆ1.09V
2Fe3+
+ 2eŌłÆ ŌåÆ 2Fe2+
(E┬░red) = +0.771V
2Fe2+
+ 2Br ŌłÆ ŌåÆ 2Fe2+
+ Br2 (E┬░cell) = ?
(E┬░cell)
= (E0ox) + (E┬░red)
=
ŌłÆ1.09 + 0.771
=
ŌłÆ0.319 V
E┬░cell is ŌĆōve; ŌłåG is +ve and the cell reaction is non spontaneous. Hence Fe3+ cannot oxidizes BrŌłÆ to Br2.
15. Is it possible to store copper sulphate in an iron vessel for a long time?
Given : ECu 2+|Cu = 0.34 V and EFe 2+|Fe = ŌłÆ0.44V .
Answer:
Given: EoCu2+|
Cu = 0.34 V
and E┬░Fe2+|Fe
= ŌłÆ 0.44V
(E┬░ox)Fe
| Fe2+ = 0.44V and (E┬░red)Cu2+ | Cu = 0.34V
The +ve emf values shows that iron will oxidise and copper will get reduced ie., the vessel will dissolve. Hence it is not possible to store copper sulphate in an iron vessel.
16. Two metals M1 and M2 have reduction potential values of -xV and +yV respectively. Which will liberate H2 and H2SO4.
Answer:
Metals having higher oxidation potential will liberate H2 from H2SO4. Hence, the metal M1 having + xV, oxidation potential will liberate H2 from H2SO4.
17. Reduction potential of two metals M and M2 are E┬║M12+|M1 = ŌłÆ 2.3V and E E┬║M22+|M2 = 0.2V Predict which one is better for coating the surface of iron. Given : E Fe2+ |Fe = ŌłÆ0.44V
Answer:
Oxidation potential of M1 is more +ve than the oxidation potential of Fe which indicates that it will prevent iron from rusting.
18. Calculate the standard emf of the cell: Cd | Cd 2+ || Cu 2+ | Cu and determine the cell reaction. The standard reduction potentials of Cu2+ | Cu and Cd2+ | Cd are 0.34V and -0.40 volts respectively. Predict the feasibility of the cell reaction.
Answer:
Cell reactions:
Oxidation
at anode : Cd(s) ŌåÆ Cd2+ (aq) + 2eŌłÆ
Reduction
at cathode: Cu2+(aq) + 2eŌłÆ ŌåÆ Cu(s)
(E┬░ox)
Cd ŌłŻ Cd2+ = 0.4V
(E┬░red)
Cu2+ | Cu= 0.34V
E┬░cell
= E┬░R - E┬░L
=
0.4 + 0.34
=
0.74V
emf
= +ve ; ŌłåG = ŌłÆve
The reaction is feasible.
19. In fuel cell H2 and O2 react to produce electricity. In the process, H2 gas is oxidised at the anode and O2 at cathode. If 44.8 litre of H2 at 25oC and 1atm pressure reacts in 10 minutes, what is average current produced? If the entire current is used for electro deposition of Cu from Cu2+ , how many grams of Cu deposited?
Oxidation at anode:
2H2(g)
+ 4OHŌłÆ (aq) ŌåÆ 4H2O (ØæÖ) + 4eŌłÆ
1
mole of hydrogen gas produces 2 moles of electrons at 25 ┬░C and 1 atm pressure,
1 mole of hydrogen gas occupies = 22.4 litres.
Ōł┤ no.of moles of hydrogen gas
produced
=
[1 mole / 22.4 litres] ├Ś 44.8 litres
=
2 moles of hydrogen
Ōł┤ 2 moles of hydrogen produces 4
moles of electron ie., 4F charge.
Q
= It
I = Q / t
=
(4F) / (10 minutes)
=
(4 ├Ś 96500 C ) / ( 10 ├Ś 60 s )
I
= 643.33 A
Electro
deposition of copper
Cu2+
(aq) + 2eŌłÆ ŌåÆ Cu(s)
2F
charge is required to deposit
1
mole of copper ie., 63.5 g
If the entire current produced in the fuel cell ie., 4F is utilized for electrolysis, then 2├Ś63.5 ie., 127.0 g copper will be deposited at cathode.
20. The same amount of electricity was passed through two separate electrolytic cells containing solutions of nickel nitrate and chromium nitrate respectively. If 2.935g of Ni was deposited in the first cell. The amount of Cr deposited in the another cell? Give : molar mass of Nickel and chromium are 58.74 and 52gm-1 respectively.
Answer:
Ni2+
(aq) + 2eŌłÆ ŌåÆ Ni(s)
Cr3+
(aq) + 3eŌłÆ ŌåÆ Cr (s)
The
above reaction indicates that 2F charge is required to deposit 58.7g of Nickel
from nickel nitrate and 3F charge is required to deposit 52 g of chromium.
Given
that 2.935 gram of Nickel is deposited
Ōł┤ The amount of charge passed
through the cell = [ 2F / 58.7g ] ├Ś 2.935g
=
0.1 F
Ōł┤ If 0.1 F charge is passed
through chromium nitrate the amount of chromium deposited
= [52g / 3F] ├Ś 0.1 F = 1.733 g
21. A copper electrode is dipped in 0.1M copper sulphate solution at 25oC . Calculate the electrode potential of copper. [Given: ECu 2+|Cu = 0.34 V ].
Answer:
Solution:
[Cu2+]
= 0.1M ;
E┬░Cu2+ ŌłŻ
Cu = 0.34 V
Ecell
= ?
Cell
reaction is
Cu2+
(aq) + 2eŌłÆ ŌåÆ Cu(s)
Ecell
= E┬░cell ŌĆō { 0.0591/n log ([Cu] / [Cu2+]) }
=
0.34 ŌĆō {(0.0591/2) log (1/0.1)}
=
0.34 ŌĆō 0.0296
Ecell
= 0.31 V
22. For the cell Mg (s) | Mg2+ (aq) || Ag+ (aq) | Ag (s), calculate the equilibrium constant at 25oC and maximum work that can be obtained during operation of cell. Given : E┬║ Mg 2+ | Mg = ŌłÆ2.37V and E┬║Ag + | Ag = 0.80V.
Answer:
Oxidation
at anode
Mg
ŌåÆ Mg2+ + 2eŌłÆ ------(1)
(E┬░ox) = 2.37 V
Reduction
at cathode
Ag++
eŌłÆ ŌåÆ Ag --------- (2)
(E┬░red) = 0.80 V
E┬░cell = (E┬░ox)anode
+ (E┬░red)cathode
=
2.37 + 0.80
=
3.17 V
Overall
reaction
Eqn
(1) + 2 ├Ś eqn (2) ŌćÆ
Mg
+ 2Ag+ ŌåÆ Mg2+ + 2Ag
ŌłåG┬░
= ŌłÆnFE┬░
=
ŌłÆ2 ├Ś 96500 ├Ś 3.17
=
ŌłÆ611.810 J
ŌłåG┬░= ŌłÆ6.12 ├Ś 105 J
ŌłåG┬░
= ŌłÆ2.303 RT log Kc
ŌćÆ log Kc = [ ŌłÆ6.12 ├Ś 105
] / [2.303 ├Ś 8.314 ├Ś 298]
Kc
= Antilog of (107.2)
23. 8.2 ├Ś1012 litres of water is available in a lake. A power reactor using the electrolysis of water in the lake produces electricity at the rate of 2 ├Ś106 CsŌłÆ1 at an appropriate voltage. How many years would it like to completely electrolyse the water in the lake. Assume that there is no loss of water except due to electrolysis.
Answer:
Hydrolysis of water
At
anode:
2H2O
ŌåÆ 4H+ + O2 + 4eŌłÆ ----------(1)
At
cathode:
2H2O
+ 2eŌłÆ ŌåÆ H2 + 2OHŌłÆ --------(2)
Overall
reaction
6H2O
ŌåÆ 4H+ + 4OHŌłÆ + 2H2+O2
(or)
Eqn (1) + (2) ├Ś 2 ŌćÆ 2H2O
ŌåÆ 2H2 + O2
Ōł┤ According to Faraday's law of
electrolysis, to electrolyse two moles of water (36g = 36 mL of H2O),
4F charge is required alternatively, when 36 mL of water is electrolysed, the
charge generated = 4 ├Ś 96500 C.
Ōł┤ When the whole water which is
available on the lake is completely electrolysed the amount of charge generated
is equal to
=
[ 4 ├Ś 96500C / 36 mL ] ├Ś 9 ├Ś 1012 L
=
[ (4 ├Ś 96500 ├Ś 9 ├Ś 1012) / (36 ├Ś 10ŌłÆ3)] ├Ś C
=
96500 ├Ś 1015 C
Ōł┤ Given that in 1 second, 2 ├Ś 106
C is generated therefore, the time required to generate
96500
├Ś 1015 C is = [ 1S / 2├Ś106C ] ├Ś 96500 ├Ś 1015 C
= 48250 ├Ś 109 S
1 years = 365 days
= 365 ├Ś 24 hours
= 365 ├Ś 24 ├Ś 60 min
= 365 ├Ś 24 ├Ś 60 ├Ś 60 sec
Ōł┤ Number of years = [ 48250 ├Ś l0
9 ] / [365 ├Ś 24 ├Ś 60 ├Ś 60]
= 1.5299 ├Ś 106 yearsŌĆā
24. Derive an expression for Nernst equation
Nernst
equation relates the cell potential and the concentration of the species
involved in an electrochemical reaction.
Consider
an electrochemical reaction is
xA
+ yB Ōćī lC + mD
The
reaction quotient Q for the above reaction is given below
Q
= [C]ØæÖ [D]m / [A]x[B]y ŌĆ”ŌĆ”ŌĆ”ŌĆ”ŌĆ”(1)
ŌłåG
= ŌłåG┬░ + RT InQ ŌĆ”ŌĆ”ŌĆ”ŌĆ”ŌĆ” (2)
The
Gibbs free energy can be related to the cell emf
ŌłåG
= ŌłÆnFEcell ;
ŌłåG┬░
= ŌłÆnFE┬░cell
Substitute
ŌłåG┬░ and Q in the eqn (2)
ŌłÆnFEcell
= ŌłÆnFE┬░cell + RT Ōäōn { [C]l[D]m
/ [A]x[B]y }
Divide
the whole eqn by (ŌłÆnF)
ŌćÆ Ecell = E┬░cell
ŌĆō { 2.303 RT / nF log. ( [C]I[D]m / [A]x[B]y
) }
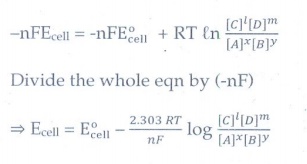
This
equation is called the Nernst equation.
25. Write a note on sacrificial protection.
It
is a method to prevent the objects from corrosion. The object is covered with
the protecting metals which is corrected more easily than the object. The metal
corrods itself but saves the object.
Eg:
Fe object is protected by Mg or Zn.
26. Explain the function of H2 - O2 fuel cell.
Fuel cell
The
energy of combustion of fuels is directly converted into electrical energy is
called the fuel cell. The general representation of a fuel cell is follows
Fuel
ŌłŻ Electrode ŌłŻ Electrolyte ŌłŻ Electrode ŌłŻ Oxidant
Fuel
- Hydrogen
Electrolyte
- Aqueous KOH
Oxidant
- Oxygen
Electrode
- Porous graphite containing Ni
Ni
and NiO serves as the inert electrodes.
Hydrogen
and oxygen gases are bubbled through the anode and cathode, respectively.
Oxidation
occurs at the anode:
2H2(g)
+ 4OHŌłÆ (aq) ŌåÆ 4H2O (Ōäō) + 4eŌłÆ
Reduction
occurs at the cathode
O2(g)
+ 2H2O(Ōäō) + 4eŌłÆ ŌåÆ 4OHŌłÆ (aq)
The
overall reaction is
2H2(g)
+ O2 (g) ŌåÆ 2H2O(Ōäō)
27. Ionic conductance at infinite dilution of Al3+ and SO42- are 189 and 160 mho cm2 equiv-1. Calculate the equivalent and molar conductance of the electrolyte Al2 (SO4 )3 at infinite dilution.
Molar conductance
╔ģ┬░m
(Al2(SO4)3) = 2╬╗┬░Al3+ + 3╬╗┬░SO42ŌłÆ
=
(2 ├Ś 189) + (3 ├Ś 160)
╔ģ┬░m
= 858 mho cm2 molŌłÆ1
Equivalent conductance
╬╗Ōł× (Al2SO4)3)
= ( 1/3 ╬╗Ōł× Al3+ ) + ( 1/2 ╬╗Ōł× SO42ŌłÆ
)
=
( 1/3 ├Ś 189) + ( 1/2 ├Ś 160 )
= 63 + 80 = 143 mho cm2 equivŌłÆ1
EVALUATE YOURSELF:
1. Calculate the
molar conductance of 0.01M aqueous KCl
solution at 25┬░C. The specific conductance of KCl at 25┬░C is 14.114 ├Ś 10 -2 SmŌłÆ1.
Given:
C
= 0.01 M
k
= 14.114 ├Ś 10ŌłÆ 2 SmŌłÆ1
╔ģm = ?
╔ģm = (K ├Ś 10ŌłÆ3) / M
=
( 14.11 ├Ś 10-2 ├Ś 10ŌłÆ3 ) / 0.01
=
(14.11 ├Ś 10-2 ├Ś 10 ŌłÆ3 ) / 10-2
╔ģm = 14.114 ├Ś 10ŌłÆ3
Sm2molŌłÆ1
2. The resistance
of 0.15N solution of an electrolyte is 50 ╬®. The specific conductance of the
solution is 2.4 SmŌłÆ1. The resistance of 0.5 N solution of the same
electrolyte measured using the same conductivity cell is 480 ╬®. Find the
equivalent conductivity of 0.5N solution of the electrolyte.
Given:
R1
= 50 ╬®
R2
= 480 ╬®
K1 = 2.4 SmŌłÆ1
K2 = ?
N1
= 0.15 N
N2
= 0.5 N
╔ģ
= [ K2(Sm ŌłÆ1) ├Ś 10- 3(gram equivalent)ŌłÆ1 m3 ] / N
We
know that
K = Cell
constant / R
Ōł┤ K2/ K1
= R1/R2
K2 = K1 ├Ś [R1/R2]
=
24 SmŌłÆ1 ├Ś (50╬® / 480╬®)
=
0.25 SmŌłÆ1
=
[ 0.25 ├Ś 10- 3 S (gram
equivalent) ŌłÆ1m2 ]
/ 0.5
╔ģ
= 5 ├Ś 10-4 Sm2 gram equivalentŌłÆ1
3. The emf of the
following cell at 25┬░C is equal to 0.34V. Calculate the reduction potential of
copper electrode.
Pt(s) ŌłŻ H2 (g, 1 atm) ŌłŻ H+ (aq, 1M) ŌłŻ ŌłŻ Cu2+ (aq, 1M) I
Cu(s)
E┬░cell
= (E┬░ox)anode + (E┬░Red)cathode
=
(E┬░ox)SHE + (E┬░Red)Cu2+
ŌłŻ Cu
=
0 + 0.34 V
E┬░cell
= 0.34V
4. Using the
calculated emf value of zinc and copper electrode, calculate the emf of the
following cell at 25 ┬░C.
Zn(s) ŌłŻ Zn2+(aq, 1M) ŌłŻ ŌłŻ Cu2+(aq, 1M) ŌłŻ Cu(s)
E┬░Zn2+ ŌłŻ Zn = ŌłÆ0.76
V
E┬░cu2+ ŌłŻ Cu = 0.34 V
E┬░cell
= E┬░R ŌłÆ E┬░L
=
E┬░Cu2+ ŌłŻ Cu
ŌłÆ E┬░Zn2+ ŌłŻ Zn
=
0.34 ŌłÆ (ŌłÆ0.76) = 1.10 V
E┬░cell = 1.10V
5. Write the
overall redox reaction which takes place in the galvanic cell,
Pt(s) ŌłŻ Fe2+(aq), Fe3+(aq) ŌłŻ ŌłŻ MnO-4
(aq) H+ (aq), Mn2+(aq) ŌłŻ Pt(s)
Oxidation
occur at anode
Fe2+
ŌåÆ Fe3+ + eŌłÆ
Reduction
occur at cathode
MnOŌłÆ4
+ 8H+ + 5eŌłÆ ŌåÆ Mn2+ + 4H2O
Overall
reaction
5Fe2+
+ MnOŌłÆ 4 + 8H+ ŌåÆ 5Fe3+ + Mn2+
+ 4H2O
6. The
electrochemical cell reaction of the Daniel cell is
Zn(s) + Cu2+
(aq) ŌåÆ Zn2+ (aq) + Cu(s)
What is the change
in the cell voltage on increasing the ion concentration in the anode
compartment by a factor 10?
For
Daniel cell
Ecell
= E┬░cell ŌĆō (0.0591/2) log ( [Zn2+] / [Cu2+] )
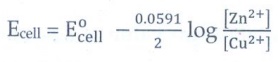
For
1 M
E
= E┬░ ŌłÆ 0.0295 log [lM/1M]
E
= E┬░ ŌłÆ 0.0295 ├Ś 0 = E┬░
For
10 M
E
= E┬░ ŌłÆ 0.0295 log [10M/1M]
E
= E┬░ ŌłÆ 0.0295 log [10]
E
= E┬░ ŌłÆ 0.0295 ├Ś 1
E
= E┬░ ŌłÆ 0.0295
On
increasing the ion concentration in anode compartment by 10 factor then the
cell voltage decreased by the value of 0.0295.
7. A solution of a
salt of metal was electrolysed for 15 minutes with a current of 0.15 amperes.
The mass of the metal deposited at the cathode is 0.783 g. Calculate the
equivalent mass of the metal.
t
= 15 min = 15 ├Ś 60 = 900 sec
I
= 0.15 A
m = 0.783 g
Z
= ?
m
= ZIt
Z
= m / It = [ 0.783g ] / [ 0.15 A ├Ś 15 ├Ś 60 sec ]
Z
= 0.0058
Related Topics