Thermodynamics of cell reactions | Electro Chemistry - Nernst equation | 12th Chemistry : UNIT 9 : Electro Chemistry
Chapter: 12th Chemistry : UNIT 9 : Electro Chemistry
Nernst equation
Nernst equation
Nernst equation is the one which relates the cell potential and the
concentration of the species involved in an electrochemical reaction. Let us
consider an electrochemical cell for which the overall redox reaction is,
xA + yB Ōåö lC + mD
The reaction quotient Q for the above reaction is given below

We have already learnt that,
ŌłåG = ŌłåGŌĆÖ + RT
lnQ .....(9.29)
The Gibbs free energy can be related to the cell emf as follows [ Ōł┤ equation
(9.24) and (9.25) ]
ŌłåG = - nFEcell;
ŌłåGo= - nFEcello
Substitute these values and Q from (9.28) in the equation (9.29)
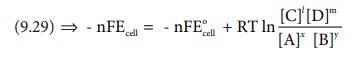
(9.29) ŌćÆ - nFEcell = - nFEocell + RT ln { [C]l[D]m / [A]x[B]y } .....(9.30)
Divide the whole equation (9.30) by (-nF)
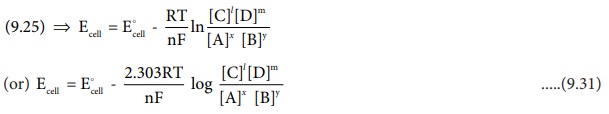
The above equation (9.31) is called the Nernst equation
At 25┬║ C
(298K), the above equation (9.31) becomes,

Let us calculate the emf of the following cell at 25oC using
Nernst equation.
Cu (s) | Cu2+ (0.25 aq, M) | | Fe3+ (0.005 aq M) |
Fe2+ (0.1 aq M) | Pt (s)
Given : ( E┬║ )Fe3+|Fe2+
= 0.77V and ( E┬║ ) Cu2+
| Cu = 0.34 V
Half reactions are
Cu (s) ŌåÆ Cu2+ (aq) + 2e- .....
(1)
2 Fe3+ ŌåÆ (aq) + 2e-
ŌåÆ 2Fe2+ (aq) ..... (2)
the overall reaction is
Cu (s) + 2 Fe3+ (aq) ŌåÆ Cu2+ (aq) + 2 Fe2+
(aq), and n = 2
Apply Nernst equation at . 25┬║C .
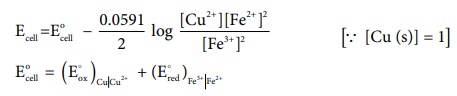
Given standard reduction potential of Cu2+ | Cu is 0.34V
(Eoox )Cu|Cu2+ = - 0.34V
(Eored) Fe3+|Fe 2+ = 0.77V
E┬║cell = - 0.34
+ 0.77
E┬║cell = 0.43V

Evaluate yourself
The electrochemical cell reaction of the Daniel cell is
Zn (s) + Cu2+ (aq) ŌåÆ Zn2+ (aq)+Cu (s)
What is the change in the cell voltage on increasing the
ion concentration in the anode compartment by a factor 10?
Related Topics