Electrochemical Cell - Measurement of electrode potential | 12th Chemistry : UNIT 9 : Electro Chemistry
Chapter: 12th Chemistry : UNIT 9 : Electro Chemistry
Measurement of electrode potential
Measurement of electrode potential
The overall redox reaction can be considered as the sum of two half reactions i.e., oxidation and reduction. Similarly, the emf of a cell can be considered as the sum of the electrode potentials at the cathode and anode,
Ecell = ( E ox )anode + ( Ered )cathode .....(9.19)
Here, ( E ox )anode represents the oxidation potential at anode and ( Ered )cathode represents the reduction potential at cathode. It is impossible to measure the emf of a single electrode, but we can measure the potential difference between the two electrodes ( E cell ) using a voltmeter. If we know the emf of any one of the electrodes which constitute the cell, we can calculate the emf of the other electrode from the measured emf of the cell using the expression (9.19). Hence, we need a reference electrode whose emf is known.
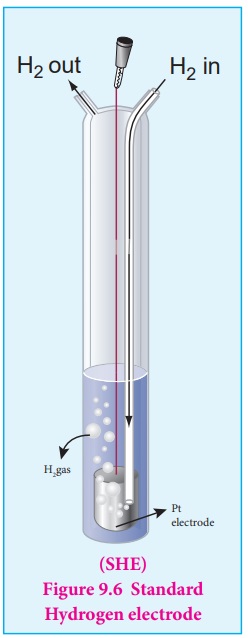
For that purpose, Standard Hydrogen Electrode (SHE) is used as the reference electrode. It has been assigned an arbitrary emf of exactly zero volt. It consists of a platinum electrode in contact with 1M HCl solution and 1 atm hydrogen gas. The hydrogen gas is bubbled through the solution at 25┬║ C as shown in the figure 9.6. SHE can act as a cathode as well as an anode. The Half cell reactions are given below.
If SHE is used as a cathode, the reduction reaction is
2H+ (aq,1M) + 2e- ŌåÆ H2 (g, 1 atm) Eo = 0 volt
If SHE is used as an anode, the oxidation reaction is
H2 (g,1 atm) ŌåÆ 2H+ (aq, 1M) + 2e- Eo = 0 volt
Illustration
Let us calculate the reduction potential of zinc electrode dipped in zinc sulphate solution using SHE.
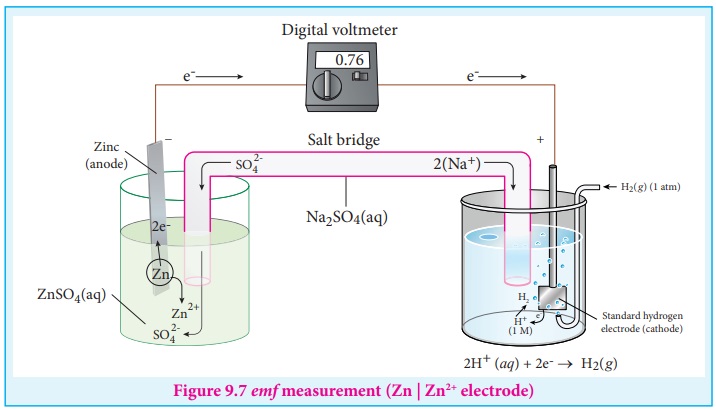
Step : 1 The following galvanic cell is constructed using SHE
Zn (s) | Zn2+ (aq, 1M) || H+ (aq, 1M) | H2 (g, 1atm) | Pt (s)
Step : 2 The emf of the above galvanic cell is measured using a volt meter. In this case, the measured emf of the above galvanic cell is 0.76V.
Calculation
We know that,
E cello = ( Eoxo )Zn|Zn2+ + ( Eredo )SHE [From equation (9.19)]
Eocell = 0.76 and (Eored )SHE = 0V .
Substitute these values in the above equation
ŌćÆ 0.76V = (Eoox)zn |Zn2+ + 0V
ŌćÆ (Eoxo )zn | Zn2+ = 0.76V
This oxidation potential corresponds to the below mentioned half cell reaction which takes place at the cathode.
Zn ŌåÆ Zn2+ + 2e- (Oxidation)
The emf for the reverse reaction will give the reduction potential
Zn2+ +2e- ŌåÆ Zn ; Eo = - 0.76V
Ōł┤ (Eored )Zn 2+ | Zn = -0.76V.
IUPAC definition
Electrode potential (E)
Electromotive force of a cell in which the electrode on the left is a standard hydrogen electrode and the electrode on the right is the electrode in question.
Standard electrode potential, E
The value of the standard emf of a cell in which molecular hydrogen under standard pressure is oxidised to solvated protons at the left hand electrode.
Related Topics