Electro Chemistry - Evaluate yourself - with Solutions | 12th Chemistry : UNIT 9 : Electro Chemistry
Chapter: 12th Chemistry : UNIT 9 : Electro Chemistry
Evaluate yourself - with Solutions
Evaluate yourself : 1
Calculate the molar conductance of 0.01M aqueous KCl solution at 25Âş C . The specific conductance of KCl at 25ÂşC is 14.114 Ă—10- 2 Sm-1 .
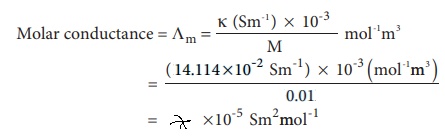
Evaluate yourself : 2
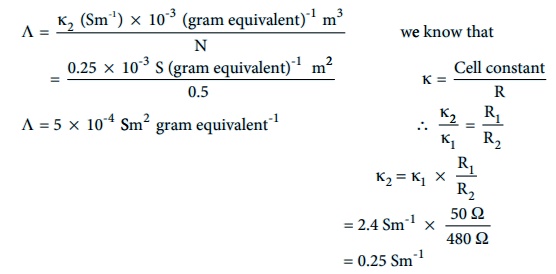
The resistance of 0.15N solution of an electrolyte is 50 Ω. The specific conductance of the solution is 2.4 Sm-1. The resistance of 0.5 N solution of the same electrolyte measured using the same conductivity cell is 480 Ω. Find the equivalent conductivity of 0.5 N solution of the electrolyte.
Given that
R1=50Ω
R2= 480Ω
Îş1 = 2.4 Sm-1
Îş2 = ?
N1 = 0.15 N
N2 = 0.5 N
Evaluate yourself
1. The emf of the following cell at 25Âş C is equal to 0.34v. Calculate the reduction potential of copper electrode.
Pt (s) | H2 (g, 1atm) | H+ (aq, 1M) || Cu2+ (aq, 1M) | Cu (s)
2. Using the calculated emf value of zinc and copper electrode, calculate the emf of the following cell at 25Âş C .
Zn (s) | Zn2+ (aq, 1M) || Cu2+ (aq, 1M) | Cu (s)
Evaluate yourself
Write the overall redox reaction which takes place in the galvanic cell,
Pt(s) | Fe2+ (aq),Fe3+ (aq) || MnO-4 (aq), H+ (aq),Mn2+ (aq) | Pt(s)
Evaluate yourself
The electrochemical cell reaction of the Daniel cell is
Zn (s) + Cu2+ (aq) → Zn2+ (aq)+Cu (s)
What is the change in the cell voltage on increasing the ion concentration in the anode compartment by a factor 10?
Evaluate yourself A solution of a salt of metal was electrolysed for 15 minutes with a current of 0.15 amperes. The mass of the metal deposited at the cathode is 0.783g. calculate the equivalent mass of the metal.
Related Topics