Thermodynamics of cell reactions | Electro Chemistry - Electrochemical series | 12th Chemistry : UNIT 9 : Electro Chemistry
Chapter: 12th Chemistry : UNIT 9 : Electro Chemistry
Electrochemical series
Electrochemical series
We have already learnt that the standard single electrode potentials are
measured using standard hydrogen electrode. The standard electrode potential at
298K for various metal - metal ion electrodes are arranged in the decreasing
order of their standard reduction potential values as shown in the figure.
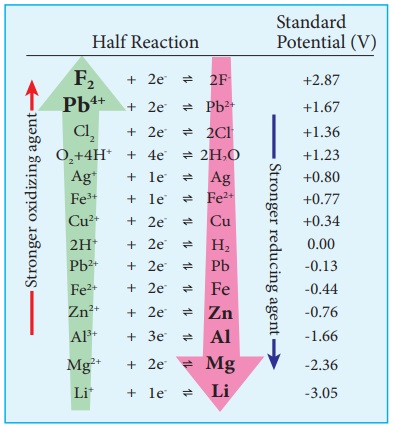
This series is called electrochemical series.
The standard reduction potential (EÂş) is a measure
of the oxidizing tendency of the species. The greater the EÂş value, greater is the tendency shown by the species
to accept electrons and undergo reduction. So higher the (EÂş) Value, lesser is the tendency to undergo corrosion
Related Topics