Thermodynamics of cell reactions | Electro Chemistry - Batteries | 12th Chemistry : UNIT 9 : Electro Chemistry
Chapter: 12th Chemistry : UNIT 9 : Electro Chemistry
Batteries
Batteries
Batteries are indispensable in the modern electronic world. For example,
Li ŌĆō ion batteries are used in cell phones, dry cell in flashlight etcŌĆ”. These
batteries are used as a source of direct current at a constant voltage. We can
classify them into primary batteries (non ŌĆō rechargeable) and secondary
batteries (rechargeable). In this section, we will briefly discuss the
electrochemistry of some batteries.
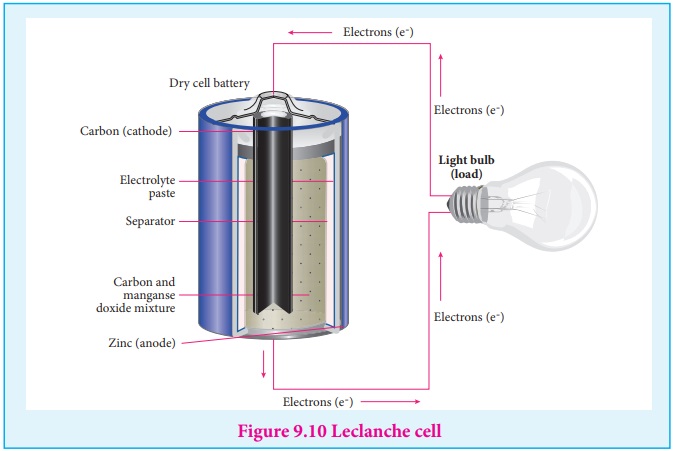
Leclanche cell
Anode : Zinc container
Cathode : Graphite rod in contact with MnO2
Electrolyte : ammonium chloride and zinc chloride in water
Emf of the cell is about 1.5V
Cell reaction
Oxidation at anode
Zn (s) ŌåÆ Zn2+
(aq)+2e- .....(1)
Reduction at cathode
2 NH4+ (aq) + 2e- ŌåÆ 2NH3(aq)
+ H2 (g) .....(2)
The hydrogen gas is oxidised to water by MnO2
H2 (g) + 2 MnO2 (s) ŌåÆ Mn2 O3 (s) + H2O
(l) .....(3)
Equation (1) + (2) + (3) gives the overall redox reaction
Zn (s) + 2NH4+ (aq) + 2 MnO2 (s) ŌåÆ Zn2+
(aq) + Mn2 O3(s) + H2O (l) + 2NH3
...... (4)
Ammonia produced at the cathode combines with Zn2+ to form a
complex ion [Zn (NH3
)4 ]2+ (aq) .
As the reaction proceeds, the concentration of NH4+ will
decrease and the aqueous NH3 will increase which lead to the
decrease in the emf of cell.
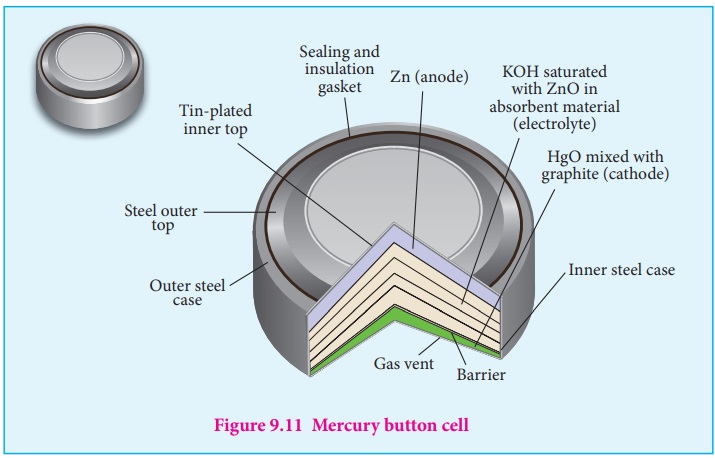
Mercury button cell
Anode : zinc amalgamated with mercury
Cathode : HgO mixed with graphite
Electrolyte : Paste of KOH and ZnO
Oxidation occurs at anode : 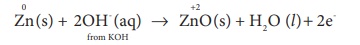
Reduction occurs at cathode : 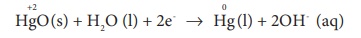
Overall reaction : Zn (s) + HgO (s) ŌåÆ ZnO (s) + Hg (l)
Cell emf : about 1.35V.
Uses : It has higher capacity and longer life. Used in pacemakers,
electronic watches, cameras etcŌĆ”
Secondary batteries
We have already learnt that the electrochemical reactions which take
place in a galvanic cell may be reversed by applying a potential slightly
greater than the emf generated by the cell. This principle is used in secondary
batteries to regenerate the original reactants. Let us understand the function
of secondary cell by considering the lead storage battery as an example
Lead storage battery
Anode : spongy lead
Cathode : lead plate bearing PbO2
Electrolyte : 38% by mass of H2 SO4 with density
1.2g / mL.
Oxidation occurs at the anode
Pb(s) ŌåÆ Pb2+
(aq)+2e- .....(1)
The Pb2+ ions combine with SO4-2 to
from PbSO4 precipitate.
Pb2+ (aq) + SO4 2ŌłÆ(aq) ŌåÆ PbSO4
(s) .....(2)
Reduction occurs at the cathode
PbO2 (s) + 4 H+ (aq) + 2e- ŌåÆ Pb2+
(aq) + 2H2O (l) ......(3)
The Pb2+ ions also combine with SO42- ions
from sulphuric acid to form precipitate.
PbSO4 Pb2+ (aq) + SO4 2ŌłÆ (aq) ŌåÆ PbSO4
(s) .....(4)
The Overall reactions is
Equation (1) + (2) + (3) + (4)
Pb (s) + PbO2 (s) + 4H + (aq) + 2SO4 2ŌłÆ (aq) ŌåÆ 2 PbSO4
(s) + 2H2O (l )
The emf of a single cell is about 2V . Usually six such cells are
combined in series to produce 12volt
The emf of the cell depends on the concentration of H2 SO4
. As the cell reaction uses SO42ŌłÆ ions,
the concentration H2 SO4 decreases. When the cell
potential falls to about 1.8V, the cell has to be recharged.
Recharge of the cell
As said earlier, a potential greater than 2V is applied across the
electrodes, the cell reactions that take place during the discharge process are
reversed. During recharge process, the role of anode and cathode is reversed
and H2 SO4 is regenerated.
Oxidation occurs at the cathode ( now act as anode)
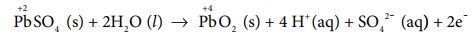
Reduction occurs at the anode (now act as cathode) PbSO4 (s)
+ 2e- ŌåÆ Pb(s) +
SO42-(aq)
Overall reaction
2PbSO4(s) + 2H2O (l ) ŌåÆ Pb (s) + PbO2 (s) + 4H+ (aq) + 2SO42-
(aq).

Thus, the overall cell reaction is exactly the reverse of the redox
reaction which takes place while discharging .
Uses:
Used in automobiles, trains, inverters etcŌĆ”
The lithium ŌĆō ion Battery
Anode : Porus graphite
Cathode : transition metal oxide such as CoO2.
Electrolyte : Lithium salt in an organic solvent
At the anode oxidation occurs
Li (s) ŌåÆ Li+
(aq) + e-
At the cathode reduction occurs
Li+ + CoO2 (s) + e- ŌåÆ Li CoO2
(s)
Overall reactions
Li (s) + CoO2 ŌåÆ LiCoO2 (s)
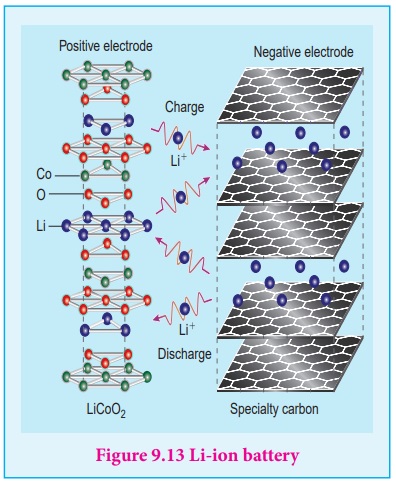
Both electrodes allow Li+ ions to move in and out of their
structures.
During discharge, the Li+ ions produced at the anode move
towards cathode through the non ŌĆō aqueous electrolyte. When a potential greater
than the emf produced by the cell is applied across the electrode, the cell
reaction is reversed and now the Li+ ions move from cathode to anode
where they become embedded on the porous graphite electrode. This is known as
intercalation.
Uses :
Used in cellular phones, laptops, computers, digital cameras, etcŌĆ”
Fuel cell
The galvanic cell in which the energy of combustion of fuels is directly
converted into electrical energy is called the fuel cell. It requires a continuous
supply of reactant to keep functioning. The general representation of a fuel
cell is follows
Fuel | Electrode | Electrolyte | Electrode | Oxidant
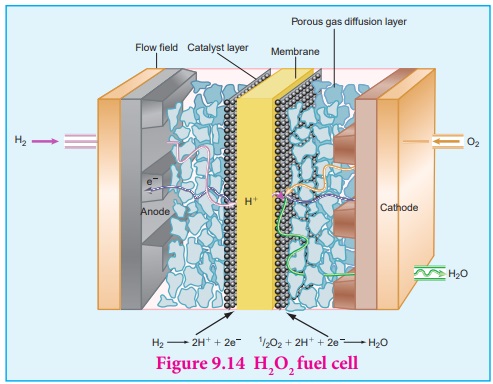
Let us understand the function of fuel cell by considering hydrogen ŌĆō
oxygen fuel cell. In this case, hydrogen act as a fuel and oxygen as an oxidant
and the electrolyte is aqueous KOH maintained at 200oC and 20 ŌĆō 40
atm. Porous graphite electrode containing Ni and NiO serves as the inert
electrodes.
Hydrogen and oxygen gases are bubbled through the anode and cathode,
respectively.
Oxidation occurs at the anode:
2H2 (g ) + 4 OHŌłÆ (aq) ŌåÆ 4 H2O
(l) + 4 eŌłÆ
Reduction occurs at the cathode O 2 (g) + 2 H2O
(l) + 4eŌłÆ ŌåÆ 4 OHŌłÆ (aq)
The overall reaction is 2H2 (g) + O2 (g) ŌåÆ 2H2O
(l)
The above reaction is the same as the hydrogen combustion reaction,
however, they do not react directly ie., the oxidation and reduction reactions
take place separately at the anode and cathode respectively like H2
- O2 fuel cell. Other fuel cells like propane ŌĆōO2 and
methane O2 have also been developed.
Related Topics