Thermodynamics of cell reactions | Electro Chemistry - Electrolytic cell and electrolysis | 12th Chemistry : UNIT 9 : Electro Chemistry
Chapter: 12th Chemistry : UNIT 9 : Electro Chemistry
Electrolytic cell and electrolysis
Electrolytic cell and electrolysis
Electrolysis is a process in which the electrical energy is used to
cause a non-spontaneous chemical reaction to occur; the energy is often used to
decompose a compound into its elements. The device which is used to carry out
the electrolysis is called the electrolytic cell. The electrochemical process
occurring in the electrolytic cell and galvanic cell are the reverse of each
other. Let us understand the function of a electrolytic cell by considering the
electrolysis of molten sodium chloride.
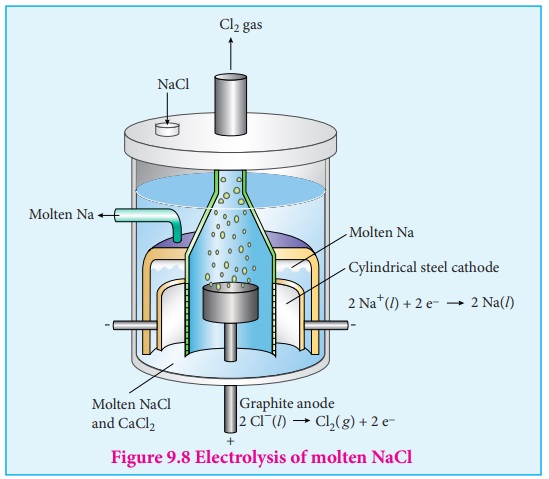
The electrolytic cell consists of two iron electrodes dipped in molten sodium chloride and they are connected to an external DC power supply via a key as shown in the figure (9.8). The electrode which is attached to the negative end of the power supply is called the cathode, and the one which attached to the positive end is called the anode. Once the key is closed, the external DC power supply drives the electrons to the cathode and at the same time pull the electrons from the anode.
Related Topics