Electro Chemistry - Electrochemical Cell | 12th Chemistry : UNIT 9 : Electro Chemistry
Chapter: 12th Chemistry : UNIT 9 : Electro Chemistry
Electrochemical Cell
Electrochemical Cell
Electrochemical cell is a device which converts chemical energy into
electrical energy and vice versa. It consists of two separate electrodes which
are in contact with an electrolyte solution. Electrochemical cells are mainly
classified into the following two types.
1. Galvanic
Cell ( Voltaic cell) : It is a device in which a spontaneous chemical
reaction generates an electric
current i.e., it converts chemical energy into electrical energy. It is
commonly known as a battery.
2. Electrolytic
cell : It is a device in which an electric current from an external source
drives a nonspontaneous reaction
i.e., it converts electrical energy into chemical energy.
1. Galvanic cell
We have already learnt in XI standard that when a zinc metal strip is
placed in a copper sulphate solution, the blue colour of the solution fades and
the copper is deposited on the zinc strip as red ŌĆō brown crust due to the
following spontaneous chemical reaction.
Zn(s) + CuSO4 (aq) ŌåÆ ZnSO4 (aq) + Cu (s)
The energy produced in the above reaction is lost to the surroundings as
heat.
In the above redox reaction, Zinc is oxidised to Zn2+ ions
and the Cu2+ ions are reduced to metallic copper. The half reactions
are represented as below.
Zn(s) ŌåÆ Zn2+(aq)
+ 2e- (oxidation)
Cu2+ (aq) + 2e- ŌåÆ Cu (s) (reduction)
If we perform the above two half reactions separately in an apparatus as
shown in figure 9.5, some of the energy produced in the reaction will be
converted into electrical energy. Let us understand the function of a galvanic
cell by considering Daniel cell as an example. It uses the above reaction for
generation of electrical energy.
The separation of half reaction is the basis for the construction of
Daniel cell. It consists of two half cells.
Oxidation half cell
A metallic zinc strip that dips into an aqueous solution of zinc
sulphate taken in a beaker, as shown in Figure 9.5.
Reduction half cell
A copper strip that dips into an aqueous solution of copper sulphate
taken in a beaker, as shown in Figure 9.5.
Joining the half cells
The zinc and copper strips are externally connected using a wire through
a switch (k) and a load (example: volt meter). The electrolytic solution
present in the cathodic and anodic compartment are connected using an inverted
U tube containing a agar-agar gel mixed with an inert electrolytes such as KCl,
Na2 SO 4 etc., The ions of inert electrolyte do not react
with other ions present in the half cells and they are not either oxidised (or)
reduced at the electrodes. The solution in the salt bridge cannot get poured
out, but through which the ions can move into (or) out of the half cells.
When the switch (k) closes the circuit, the electrons flows from zinc
strip to copper strip.
This is due to the following redox reactions which are taking place at
the respective electrodes.
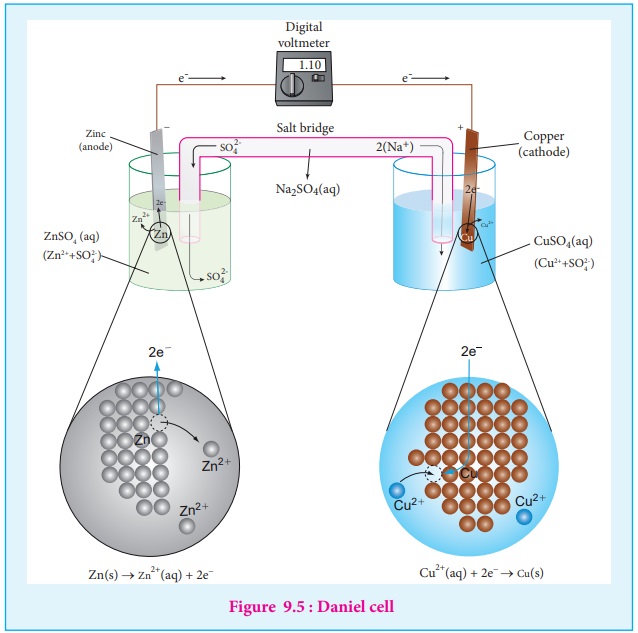
Anodic oxidation
The electrode at which the oxidation occurs is called the anode. In
Daniel cell, the oxidation take place at zinc electrode, i.e., zinc is oxidised
to Zn2+ ions by loosing its electrons. The Zn2+ ions
enter the solution and the electrons enter the zinc metal, then flow through
the external wire and then enter the copper strip. Electrons are liberated at
zinc electrode and hence it is negative ( - ve).
Zn(s) ŌåÆ Zn2+
(aq) + 2e- (loss
of electron-oxidation)
Cathodic reduction
As discussed earlier, the electrons flow through the circuit from zinc
to copper, where the Cu2+ ions in the solution accept the electrons,
get reduced to copper and the same get deposited on the electrode. Here, the
electrons are consumed and hence it is positive (+ve).
Cu2+ (aq) + 2e- ŌåÆ Cu (s) (gain of electron-reduction)
Salt bridge
The electrolytes present in two half cells are connected using a salt
bridge. We have learnt that the anodic oxidation of zinc electrodes results in
the increase in concentration of Zn2+ in solution. i.e., the
solution contains more number of Zn2+ ions as compared to SO42-
and hence the solution in the anodic compartment would become positively
charged. Similarly, the solution in the cathodic compartment would become
negatively charged as the Cu2+ ions are reduced to copper i.e., the
cathodic solution contain more number of SO42- ions
compared to Cu2+ .
To maintain the electrical neutrality in both the compartments, the non
reactive anions Cl- (from KCl taken in the salt bridge) move from
the salt bridge and enter into the anodic compartment, at the same time some of
the K+ ions move from the salt bridge into the cathodic compartment.
Completion of circuit
Electrons flow from the negatively charged zinc anode into the
positively charged copper cathode through the external wire, at the same time,
anions move towards anode and cations are move towards the cathode compartment.
This completes the circuit.
Consumption of Electrodes
As the Daniel cell operates, the mass of zinc electrode gradually
decreases while the mass of the copper electrode increases and hence the cell
will function until the entire metallic zinc electrode is converted in to Zn2+
or the entire Cu2+ ions are converted in to metallic copper.
Unlike Daniel cell, in certain cases, the reactants (or) products cannot
serve as electrodes and in such cases inert electrode such as graphite (or)
platinum is used which conducts current in the external circuit.
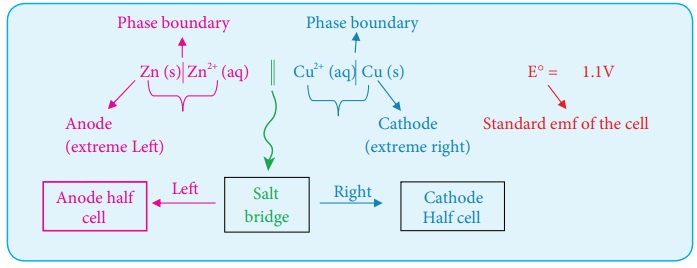
2. Galvanic cell notation
The galvanic cell is represented by a cell diagram, for example, Daniel
cell is represented as
Zn (s) | Zn2+ (aq) || Cu2+ (aq) |Cu (s)
In the above notation, a single vertical bar (|) represents a phase
boundary and the double vertical bar (||) represents the salt bridge.
The anode half cell is written on the left side of the salt bridge and
the cathode half cell on the right side.
The anode and cathode are written on the extreme left and extreme right,
respectively.
The emf of the cell is written on the right side after cell diagram.
Example
The net redox reaction of a galvanic cell is given below
2 Cr (s) + 3Cu2+ (aq) ŌåÆ 2Cr3+ (aq) + 3Cu (s)
Write the half reactions and describe the cell using cell notation.
Anodic oxidation 2Cr (s) ŌåÆ 2Cr3+ (aq) + 6e- .....(1)
Cathodic reduction : 3Cu2+ (aq) + 6e- ŌåÆ 3 Cu
(s) .....(2)
Cell Notation is
Cr (s) | Cr3+ (aq) || Cu2+ (aq) | Cu(s)
emf of a Cell
We have learnt that when two half cells of a Daniel cell are connected,
a spontaneous redox reaction will take place which results in the flow of
electrons from anode to cathode. The force that pushes the electrons away from
the anode and pulls them towards cathode is called the electromotive force
(emf) (or) the cell potential. The SI unit of cell potential is the volt (v).
When there is one volt difference in electrical potential between the
anode and cathode, one joule of energy is released for each columb of charge
that moves between them.
i.e., 1J = 1C ├Ś 1V .....(9.18)
The cell voltage depends on the nature of the electrodes, the
concentration of the electrolytes and the temperature at which the cell is
operated. For example
At, 25 C , The emf of the below mentioned Daniel cell is 1.107 Volts
Zn (s) | Zn2+ (aq,1M) || Cu2+ (aq,1M) | Cu ( s ) E0
= 1.107 V
Measurement of electrode potential
The overall redox reaction can be considered as the sum of two half reactions i.e., oxidation and reduction. Similarly, the emf of a cell can be considered as the sum of the electrode potentials at the cathode and anode,
Ecell = ( E ox
)anode + ( Ered
)cathode .....(9.19)
Here, ( E ox
)anode represents
the oxidation potential at anode and ( Ered )cathode represents the reduction potential at cathode. It
is impossible to measure the emf of a single electrode, but we can measure the
potential difference between the two electrodes ( E cell ) using a voltmeter. If we know the emf of any one
of the electrodes which constitute the cell, we can calculate the emf of the
other electrode from the measured emf of the cell using the expression (9.19).
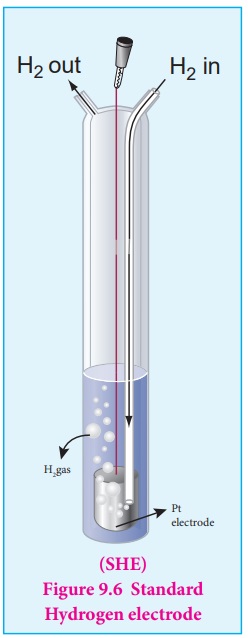
Hence, we need a reference electrode whose emf is known.
For that purpose, Standard Hydrogen Electrode (SHE) is used as the reference electrode. It has been assigned an
arbitrary emf of exactly zero volt. It consists of a platinum electrode in
contact with 1M HCl solution and 1 atm hydrogen gas. The hydrogen gas is
bubbled through the solution at 25┬║ C as
shown in the figure 9.6. SHE can act as a cathode as well as an anode. The Half
cell reactions are given below.
If SHE is used as a cathode, the reduction reaction is
2H+ (aq,1M) + 2e- ŌåÆ H2 (g, 1 atm) Eo = 0 volt
If SHE is used as an anode, the oxidation reaction is
H2 (g,1 atm) ŌåÆ 2H+
(aq, 1M) + 2e- Eo = 0 volt
Illustration
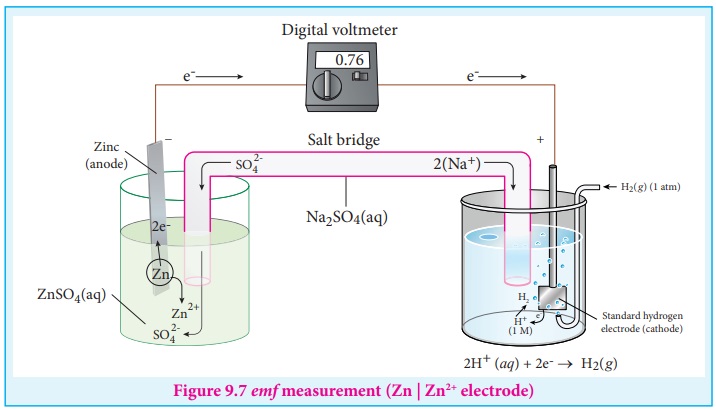
Let us calculate the reduction potential of zinc electrode dipped in
zinc sulphate solution using SHE.
Step : 1 The following galvanic cell is
constructed using SHE
Zn (s) | Zn2+ (aq, 1M) || H+ (aq, 1M) | H2
(g, 1atm) | Pt (s)
Step : 2 The emf of the above galvanic cell is
measured using a volt meter. In this case, the measured emf of the above galvanic cell
is 0.76V.
Calculation
We know
that,
E cello = ( Eoxo )Zn|Zn2+ + ( Eredo
)SHE [From equation (9.19)]
Eocell = 0.76 and (Eored )SHE
= 0V .
Substitute these values in the above equation
ŌćÆ 0.76V = (Eoox)zn |Zn2+
+ 0V
ŌćÆ (Eoxo )zn | Zn2+ = 0.76V
This oxidation potential corresponds to the below mentioned half cell
reaction which takes place at the cathode.
Zn ŌåÆ Zn2+
+ 2e- (Oxidation)
The emf for the reverse reaction will give the reduction potential
Zn2+ +2e- ŌåÆ Zn ; Eo = - 0.76V
Ōł┤ (Eored )Zn 2+ | Zn
= -0.76V.
IUPAC definition
Electrode
potential (E)
Electromotive force of a cell in which the electrode on the left is a
standard hydrogen electrode and the electrode on the right is the electrode in
question.
Standard
electrode potential, E
The value of the standard emf of a cell in which molecular hydrogen
under standard pressure is oxidised to solvated protons at the left hand
electrode.
Evaluate yourself
1. The emf of the following cell at 25┬║ C is equal to 0.34v. Calculate the reduction potential of
copper electrode.
Pt (s) | H2 (g, 1atm) | H+ (aq, 1M) ||
Cu2+ (aq, 1M) | Cu (s)
2. Using the calculated emf value of zinc and copper electrode,
calculate the emf of the following cell at 25┬║ C .
Zn (s) | Zn2+ (aq, 1M) || Cu2+ (aq,
1M) | Cu (s)
Evaluate yourself
Write the overall redox reaction which takes place in the
galvanic cell,
Pt(s) | Fe2+ (aq),Fe3+ (aq) || MnO-4
(aq), H+ (aq),Mn2+ (aq) | Pt(s)
Related Topics