Thermodynamics of cell reactions | Electro Chemistry - Corrosion | 12th Chemistry : UNIT 9 : Electro Chemistry
Chapter: 12th Chemistry : UNIT 9 : Electro Chemistry
Corrosion
Corrosion
We are familiar with the rusting of iron. Have you ever noticed a green
film formed on copper and brass vessels?. In both, the metal is oxidised by
oxygen in presence of moisture. This redox process which causes the
deterioration of metal is called corrosion. As the corrosion of iron causes
damages to our buildings, bridges etc....it is important to know the chemistry
of rusting and how to prevent it. Rusting of iron is an electrochemical
process.
Electrochemical mechanism of corrosion
The formation of rust requires both oxygen and water. Since it is an
electrochemical redox process, it requires an anode and cathode in different places
on the surface of iron. The iron surface and a droplet of water on the surface
as shown in figure (9.15) form a tiny galvanic cell. The region enclosed by
water is exposed to low amount of oxygen and it acts as the anode. The
remaining area has high amount of oxygen and it acts as cathode. So based on
the oxygen content, an electro chemical cell is formed. corrosion occurs at the
anode i,e,. in the region enclosed by the water as discussed below.
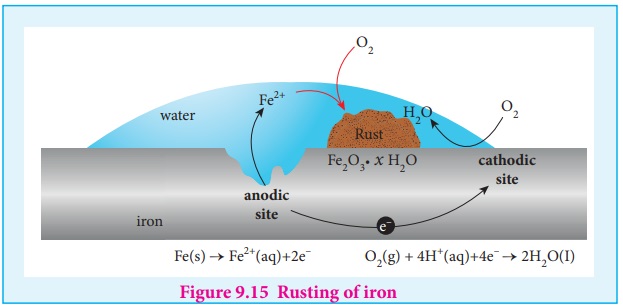
At anode (oxidation): Iron dissolves in the anode region
2Fe(s) ŌåÆ 2Fe2+
(aq) + 4e- E = 0.44V.
The electrons move through the iron metal from the anode to the cathode
area where the oxygen dissolved in water, is reduced to water.
At Cathode (reduction)
The reaction of atmospheric carbon dioxide with water gives carbonic
acid which furnishes the H+ ions for reduction.
O2 (g) + 4H+ (aq) + 4e- ŌåÆ 2H2O
(l ) E
= 1.23V
The electrical circuit is completed by the migration of ions through
water droplet.
The overall redox reactions is,
2Fe(s) + O2(g) + 4H+ (aq) ŌåÆ 2Fe2+
(aq) + 2H2O(l ) E = 0.444 +1.23 = 1.67V
The positive emf value indicates that the reaction is spontaneous.
Fe2+ ions are further oxidised to Fe3+ , which on
further reaction with oxygen to form rust.
4Fe2+ (aq)+O2(g)+4H+ (aq) ŌåÆ 4Fe3+
(aq)+2H2O(l)
2Fe3+ (aq)+4H2O(l) ŌåÆ Fe2O3.H2O(s) + 6H+
(aq)
Other metals such as aluminium, copper and silver also undergo
corrosion, but at a slower rate than iron. For example, let us consider the
reduction of aluminium,
Al(s) ŌåÆ Al3+ (aq)+3eŌłÆ
Al3+ , which reacts with oxygen in air to forms a protective coating of Al2O3
. This coating act as a protective film for the inner surface. So,further
corrosion is prevented.
Protection of metals form corrosion
This can be achieved by the following methods.
i. Coating metal surface by paint.
ii. Galvanizing - by coating with another metal
such as zinc. zinc is stronger reducing agent than iron and hence it can be
more easily corroded than iron. i.e., instead of iron, the zinc is oxidised.
iii. Cathodic protection - In this technique,
unlike galvanising the entire surface of the metal to be protected need not be
covered with a protecting metal. Instead, metals such as Mg or zinc which is
corroded more easily than iron can be used as a sacrificial anode and the iron
material acts as a cathode. So iron is protected, but Mg or Zn is corroded.
Passivation - The metal is treated with strong
oxidising agents such as concentrated HNO3. As a result, a protective
oxide layer is formed on the surface of metal.
Alloy formation - The oxidising tendency of iron can be
reduced by forming its alloy with other more anodic metals.
Example, stainless steel - an alloy of Fe and Cr .
Related Topics