Question with Answers, Solution | Electro Chemistry - Numerical Problems | 12th Chemistry : UNIT 9 : Electro Chemistry
Chapter: 12th Chemistry : UNIT 9 : Electro Chemistry
Numerical Problems
Short Answer Questions
Example
A conductivity cell has two platinum electrodes separated by a distance 1.5 cm and the cross sectional area of each electrode is 4.5 sq cm. Using this cell, the resistance of 0.5 N electrolytic solution was measured as 15 Ōä” . Find the specific conductance of the solution.
Solution
l = 1 5. cm = 1.5├Ś10-2 m
A = 4.5 cm2 = 4.5├Ś(10-4)m2
R = 15Ōä”
╬║ = 1/R (l/A)
╬║ = [ 1/15Ōä” ] ├Ś [1.5├Ś10-2 m / 4.5├Ś(10-4)m2 ]
= 2.22 Sm-1
Example
Calculate the molar conductance of 0.025M aqueous solution of calcium chloride at 25┬║ C. The specific conductance of calcium chloride is 12.04 ├Ś 10-2 Sm-1.
Molar conductance = ╬ø m =
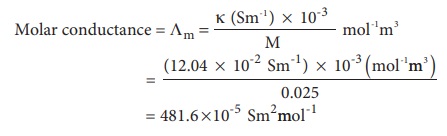
= 481.6 ├Ś10-5 Sm2mol-1
Example
The resistance of a conductivity cell is measured as 190 Ōä” using 0.1M KCl solution (specific conductance of 0.1M KCl is 1.3 Sm-1 ) . When the same cell is filled with 0.003M sodium chloride solution, the measured resistance is 6.3KŌä”. Both these measurements are made at a particular temperature. Calculate the specific and molar conductance of NaCl solution.
Given that
╬║ = 1.3 Sm-1 (for 0.1M KCl solution)
R = 190 Ōä”
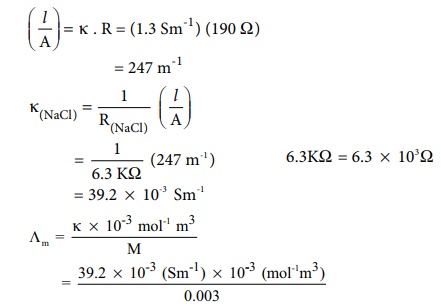
╬øm = 13.04 ├Ś 10-3 Sm2 mol-1
Example
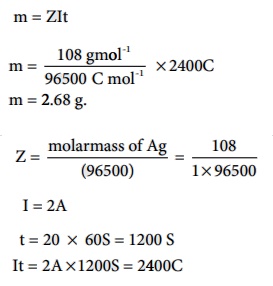
A solution of silver nitrate is electrolysed for 20 minutes with a current of 2 amperes. Calculate the mass of silver deposited at the cathode.
Electrochemical reaction at cathode is Ag+ +e- ŌåÆ Ag (reduction)
Book Back Questions and Answers
8. The
conductivity of a 0.01M solution of a 1 :1 weak electrolyte at 298K is 1.5 ├Ś10-4
S cmŌłÆ1.
i) molar
conductivity of the solution
ii) degree
of dissociation and the dissociation constant of the weak electrolyte
Given
that
╬╗┬║cation = 248.2 S cm2 molŌłÆ1
╬╗┬║cation = 51.8 S cm2 molŌłÆ1
Answer:
Given
C = 0.01M
╬╗┬║cation = 248.2 S cm2
molŌłÆ1
K= 1.5 ├Ś 10ŌłÆ4
S cmŌłÆ1
╬╗┬║anion = 51.8 S cm2
molŌłÆ1.
1. Molar
conductivity
K = 1.5 ├Ś10ŌłÆ4
S cmŌłÆ1
╬ø┬║m =
K(smŌłÆ1 ) ├Ś10ŌłÆ3 / C (in M) mol-1 m3
= (1.5 ├Ś102
├Ś10ŌłÆ3 ) / 0.01 S mol ŌłÆ1 m2 1 cmŌłÆ1 = 102mŌłÆ1
= 1.5 ├Ś10ŌłÆ3
S m2 molŌłÆ1 = 1.5 ├Ś102
2. Degree of
dissociation ╬▒ = ╬ø┬║/╬ø┬║Ōł×
╬øoŌł× =╬╗ocation +╬╗aniono
= (248.2 + 51.8) S cm2 molŌłÆ1
= 300 S cm2
molŌłÆ1
= 300 ├Ś10ŌłÆ14 s m2
molŌłÆ1
╬▒ = 1.5 ├Ś10ŌłÆ3 S m2
molŌłÆ1 / 300├Ś10ŌłÆ4 S m2
molŌłÆ1
╬▒ = 0.05
Ka = ╬▒2c
/ 1-╬▒
= [ (0.05)2
(0.01) ] / 1-0.05
= 25 ├Ś10ŌłÆ4
├Ś10ŌłÆ2 / 95 ├Ś10ŌłÆ2
= 0.26 ├Ś10ŌłÆ4
= 2.6 ├Ś10ŌłÆ5.
13. A
current of 1.608A is passed through 250 mL of 0.5M solution of copper sulphate
for 50 minutes. Calculate the strength of Cu2+ after electrolysis
assuming volume to be constant and the current efficiency is 100%.
Answer:
Given
I = 1.608A; t = 50
min = 50 ├Ś 60
= 3000S
╬Ę = 100%
V=250 mL
C=0.5M
Calculate the number of faradays of
electricity passed through the CuSO4 solution
ŌćÆ Q=It
Q = 1.608 ├Ś3000
Q = 4824C
Ōł┤ number of Faradays of electicity = 4824 C / 96500C = 0.05F
Electrolysis of
CuSO4
Cu2+
(aq)+2eŌłÆ ŌåÆ Cu(s).
The above equation
shows that 2F electricity will deposit 1 mole of Cu2+ to Cu.
Ōł┤0.05F electricity will
deposit 1 mol /2F ├Ś 0 .05F = 0.025 mol
Initial number of
molar of Cu2+ in 250 ml of solution = (0.5 / 1000 mL) ├Ś 250mL
= 0.125 mol
Ōł┤ number of moles of Cu2+ after electrolysis
= 0.125 - 0.025
= 0.1 mol
Ōł┤ Concentration of Cu2+ = [ 0.1 mol/ 250 mL ] ├Ś1000 mL
=0.4M
14. Can
Fe3+ oxidises bromide to bromine under standard conditions?
Given: EFe
3+|Fe2+ = 0.771
EBr2|BrŌłÆ = 1.09V.
Answer:
Required half cell reaction
2 Br- ŌåÆ Br2 + 2eŌłÆ
( Eoox
)= ŌłÆ1.09V
2 Fe3+ + 2eŌłÆ ŌåÆ 2Fe2+
( Eored
)= +0.771V
2Fe3+ + 2BrŌłÆ ŌåÆ 2Fe2+ +Br2
( Eocell
)= ?
Eocell
= ( Eoox )+ ( Eored
)
= ŌłÆ1.09 + 0.771
ŌłÆ0.319V
Ecell is
ŌĆō ve; ŌłåG is +ve and the
cell reaction is non spontaneous. Hence Fe3+ cannot oxidises Br-
to Br2
15. Is it
possible to store copper sulphate in an iron vessel for a long time?
Given : ECu
2+|Cu = 0.34 V and EFe 2+|Fe = ŌłÆ0.44V .
Answer:
( Eoxo )Fe|Fe2+ = 0.44V and ( Eredo
)Cu 2+|Cu = 0.34V .
These +ve emf
values shows that iron will oxidise and copper will get reduced i.e., the
vessel will dissolve.
Hence it is not
possible to store copper sulphate in an iron vessel.
16. Two
metals M1 and M2 have reduction potential values of -xV and +yV respectively. Which will
liberate H2 and H2SO4.
Answer:
Metals having
higher oxidation potential will liberate H2 from H2 SO4
. Hence, the metal M1 having + xV, oxidation potential will liberate
H2 from H2SO4 .
17.
Reduction potential of two metals M and M2 are E┬║M12+|M1 = ŌłÆ 2.3V
and E E┬║M22+|M2 = 0.2V Predict which one is better for coating the
surface of iron. Given : E Fe2+ |Fe = ŌłÆ0.44V
Answer:
oxidation potential
of M1 is more +ve than the oxidation potential of Fe which indicates
that it will prevent iron from rusting
18. Calculate
the standard emf of the cell: Cd | Cd 2+ || Cu 2+
| Cu and determine the cell reaction. The standard reduction potentials of Cu2+
| Cu and Cd2+ | Cd are 0.34V and -0.40 volts respectively.
Predict the feasibility of the cell reaction.
Answer:
Cell reactions:
Oxidation at anode:
Cd (s) ŌåÆ Cd2+ (aq) + 2eŌłÆ
( Eoox)
Cu|Cd2+ = 0.4V
Reduction at
cathode: Cu2+ (aq) + 2e- ŌåÆ Cu (s)
( Eored
)Cu2+|Cu = 0.34V
Cd ( s ) + 2e- ŌåÆ Cd2+ (aq) + Cu(s)
Ecell = ( Eoox
)+ ( Eredo
)cathode
= 0.4 + 0.34
= 0.74V.
emf is +ve, so ŌłåG is (-)ve, the
reaction is feasible.
19. In
fuel cell H2 and O2 react to produce electricity. In the
process, H2 gas is oxidised at the anode and O2 at
cathode. If 44.8 litre of H2 at 25oC and 1atm pressure
reacts in 10 minutes, what is average current produced? If the entire current
is used for electro deposition of Cu from Cu2+ , how many grams of
Cu deposited?
Answer:
Oxidation at anode:
2H2 (g)
+ 4OH- (aq) ŌåÆ 4H2O
(l) + 4eŌłÆ
1 mole of hydrogen
gas produces 2 moles of electrons at 25┬║ C and 1 atm pressure, 1 mole of hydrogen gas occupies = 22.4
litres
Ōł┤ no. of moles of hydrogen gas produced = [1 mole / (22.4
litres)] ├Ś 44.8 litres
= 2 moles of hydrogen
Ōł┤ 2 of moles of hydrogen produces 4 moles of electron i.e., 4F
charge.
We know that Q= It
I= Q/t
= 4F / 10 mins
= 4├Ś96500 C / 10├Ś60
s
I=643.33 A
Electro deposition
of copper
Cu2+
(aq)+2e- ŌåÆ Cu(s)
2F charge is
required to deposit
1 mole of copper
i.e., 63.5 g
If the entire
current produced in the fuel cell ie., 4 F is utilised for electrolysis, then
2├Ś 63.5 i.e., 127.0 g copper will be deposited at cathode.
20. The
same amount of electricity was passed through two separate electrolytic cells
containing solutions of nickel nitrate and chromium nitrate respectively. If
2.935g of Ni was deposited in the first cell. The amount of Cr deposited in the
another cell? Give : molar mass of Nickel and chromium are 58.74 and 52gm-1
respectively.
Answer:
Ni2+ (aq) + 2e- ŌåÆ Ni (s)
Cr2+
(aq)+3eŌłÆ ŌåÆ Cr (s)
The above reaction
indicates that 2F charge is required to deposit 58.7g of Nickel form nickel
nitrate and 3F charge is required to deposit 52g of chromium.
Given that 2.935
gram of Nickel is deposited
Ōł┤The amount of charge passed through the cell = (2F/58.7 g) ├Ś
2.935g
= 0.1F
Ōł┤ if 0.1F charge is passed through chromium nitrate the amount of
chromium deposited = 52g/3F ├Ś 0.1F
= 1.733g
21. A
copper electrode is dipped in 0.1M copper sulphate solution at 25oC
. Calculate the electrode potential of copper. [Given: ECu 2+|Cu
= 0.34 V
].
Answer:
Given that
[ Cu2+]
= 0.1M
E┬║Cu2+ |Cu = 0.34
Ecell = ?
Cell reaction is
Cu2+
(aq) + 2eŌłÆ ŌåÆ Cu (s)
Ecell =
Eo ŌĆō [0.0591/n] log {[Cu] [Cu2+]}
= 0.34 ŌĆō [0.0591/2]
log (1/0.1)
= 0.34 ŌłÆ0.0296
= 0.31V
22. For the
cell Mg (s) | Mg2+ (aq) || Ag+ (aq) | Ag (s), calculate the equilibrium constant
at 25oC and maximum work that
can be obtained during operation of cell. Given : E┬║ Mg 2+ | Mg = ŌłÆ2.37V and
E┬║Ag + | Ag = 0.80V.
Answer:
oxidation at anode
Mg ŌåÆ Mg2+
+2e- ..............
(1) ( E┬║xo) = 2.37V
Reduction at
cathode
Ag+ + e-
ŌåÆ Ag ........... (2) ( E┬║red) = 0.80V
Ōł┤ E┬║cell = ( E┬║ox )anode + ( E┬║red )cathode
= 2.37+0.80
= 3.17V
Overall reaction
Equation (1) + 2 ├Ś equation ( 2 ) ŌćÆ
Mg+ 2Ag2+ ŌåÆ Mg2+ + 2Ag
ŌłåG = -nfE
=ŌłÆ2 ├Ś 96500 ├Ś 3.17
=ŌłÆ611810 J
ŌłåGo = ŌłÆ6.12 ├Ś105 J
W = 6.12 ├Ś105 J
ŌłåGo =ŌłÆ2.303 RT logKC
ŌłåGo =ŌłÆ2.303 RT logKC
ŌćÆ log Kc = (6.12 ├Ś105)
/ (2.303 ├Ś 8.314 ├Ś 298)
Kc = Antilog of (107.2)
23. 8.2 ├Ś1012
litres of water is available in a lake. A power reactor using the electrolysis
of water in the lake produces electricity at the rate of 2 ├Ś106
CsŌłÆ1 at an
appropriate voltage. How many years would it like to completely electrolyse the
water in the lake. Assume that there is no loss of water except due to
electrolysis.
Answer:
Electrolysis of
water
At anode:
2H2O ŌåÆ 4H+ + O2 + 4eŌłÆ ......... (1)
At cathode:
2H2 O+2eŌłÆ ŌåÆ H2 + 2OH-
Overall reaction 6H2O
ŌåÆ 4H+ + 4OH- + 2H2 + O2
(or)
Equation (1) +(2) ├Ś2 ŌćÆ 2H2 O ŌåÆ 2H2 + O2
According to faradays Law of electrolysis, to electrolyse two
mole of Water (36g = 36 mL of H2O), 4F charge is required
alternatively, when 36 mL of water is electrolysed, the charge generated = 4 ├Ś 96500 C.
When the whole
water which is available on the lake is completely electrolysed the amount of
charge generated is equal to (4 ├Ś 96500 C / 36 mL) ├Ś 9 ├Ś1012 L
= ( 4 ├Ś 96500 ├Ś 9 ├Ś1012 ) / ( 36 ├Ś10ŌłÆ3 ) C
= 96500 ├Ś1015 C
Ōł┤Given that in 1 second, 2 ├Ś106 C is generated therefore, the time required to
generate
96500 ├Ś1015 C is = [ 1 S/ 2 ├Ś106C] ├Ś 96500 ├Ś1015 C
= 48250 ├Ś109 S
1 year = 365 days
= 365 ├Ś 24 hours
= 365├Ś24 ├Ś 60 min
1 year = 365├Ś24 ├Ś
60 ├Ś 60 sec.
Ōł┤ Number of years = 48250 ├Ś109 / 365├Ś24├Ś60├Ś60
= 1.5299 ├Ś106 years
Related Topics