Electrochemical Cell - Galvanic cell | 12th Chemistry : UNIT 9 : Electro Chemistry
Chapter: 12th Chemistry : UNIT 9 : Electro Chemistry
Galvanic cell
Galvanic cell
We have already learnt in XI standard that when a zinc metal strip is placed in a copper sulphate solution, the blue colour of the solution fades and the copper is deposited on the zinc strip as red – brown crust due to the following spontaneous chemical reaction.
Zn(s) + CuSO4 (aq) → ZnSO4 (aq) + Cu (s)
The energy produced in the above reaction is lost to the surroundings as heat.
In the above redox reaction, Zinc is oxidised to Zn2+ ions and the Cu2+ ions are reduced to metallic copper. The half reactions are represented as below.
Zn(s) → Zn2+(aq) + 2e- (oxidation)
Cu2+ (aq) + 2e- → Cu (s) (reduction)
If we perform the above two half reactions separately in an apparatus as shown in figure 9.5, some of the energy produced in the reaction will be converted into electrical energy. Let us understand the function of a galvanic cell by considering Daniel cell as an example. It uses the above reaction for generation of electrical energy.
The separation of half reaction is the basis for the construction of Daniel cell. It consists of two half cells.
Oxidation half cell
A metallic zinc strip that dips into an aqueous solution of zinc sulphate taken in a beaker, as shown in Figure 9.5.
Reduction half cell
A copper strip that dips into an aqueous solution of copper sulphate taken in a beaker, as shown in Figure 9.5.
Joining the half cells
The zinc and copper strips are externally connected using a wire through a switch (k) and a load (example: volt meter). The electrolytic solution present in the cathodic and anodic compartment are connected using an inverted U tube containing a agar-agar gel mixed with an inert electrolytes such as KCl, Na2 SO 4 etc., The ions of inert electrolyte do not react with other ions present in the half cells and they are not either oxidised (or) reduced at the electrodes. The solution in the salt bridge cannot get poured out, but through which the ions can move into (or) out of the half cells.
When the switch (k) closes the circuit, the electrons flows from zinc strip to copper strip.
This is due to the following redox reactions which are taking place at the respective electrodes.
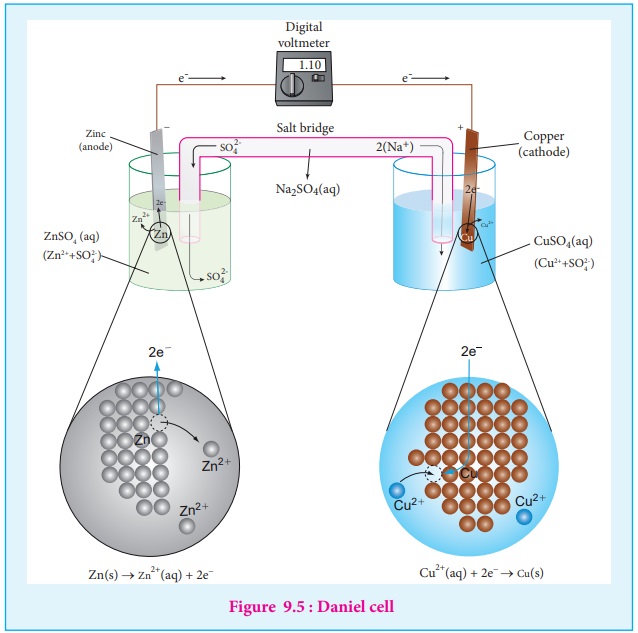
Anodic oxidation
The electrode at which the oxidation occurs is called the anode. In Daniel cell, the oxidation take place at zinc electrode, i.e., zinc is oxidised to Zn2+ ions by loosing its electrons. The Zn2+ ions enter the solution and the electrons enter the zinc metal, then flow through the external wire and then enter the copper strip. Electrons are liberated at zinc electrode and hence it is negative ( - ve).
Zn(s) → Zn2+ (aq) + 2e- (loss of electron-oxidation)
Cathodic reduction
As discussed earlier, the electrons flow through the circuit from zinc to copper, where the Cu2+ ions in the solution accept the electrons, get reduced to copper and the same get deposited on the electrode. Here, the electrons are consumed and hence it is positive (+ve).
Cu2+ (aq) + 2e- → Cu (s) (gain of electron-reduction)
Salt bridge
The electrolytes present in two half cells are connected using a salt bridge. We have learnt that the anodic oxidation of zinc electrodes results in the increase in concentration of Zn2+ in solution. i.e., the solution contains more number of Zn2+ ions as compared to SO42- and hence the solution in the anodic compartment would become positively charged. Similarly, the solution in the cathodic compartment would become negatively charged as the Cu2+ ions are reduced to copper i.e., the cathodic solution contain more number of SO42- ions compared to Cu2+ .
To maintain the electrical neutrality in both the compartments, the non reactive anions Cl- (from KCl taken in the salt bridge) move from the salt bridge and enter into the anodic compartment, at the same time some of the K+ ions move from the salt bridge into the cathodic compartment.
Completion of circuit
Electrons flow from the negatively charged zinc anode into the positively charged copper cathode through the external wire, at the same time, anions move towards anode and cations are move towards the cathode compartment. This completes the circuit.
Consumption of Electrodes
As the Daniel cell operates, the mass of zinc electrode gradually decreases while the mass of the copper electrode increases and hence the cell will function until the entire metallic zinc electrode is converted in to Zn2+ or the entire Cu2+ ions are converted in to metallic copper.
Unlike Daniel cell, in certain cases, the reactants (or) products cannot serve as electrodes and in such cases inert electrode such as graphite (or) platinum is used which conducts current in the external circuit.
Related Topics