Electro Chemistry - Galvanic cell notation | 12th Chemistry : UNIT 9 : Electro Chemistry
Chapter: 12th Chemistry : UNIT 9 : Electro Chemistry
Galvanic cell notation
Galvanic cell notation
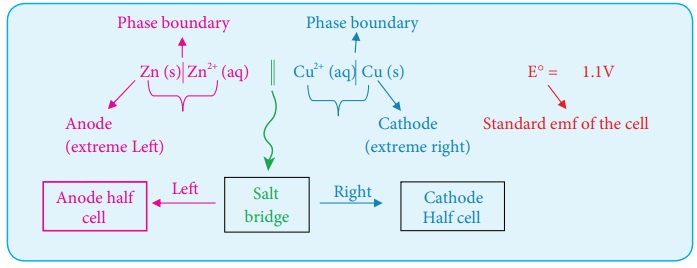
The galvanic cell is represented by a cell diagram, for example, Daniel cell is represented as
Zn (s) | Zn2+ (aq) || Cu2+ (aq) |Cu (s)
In the above notation, a single vertical bar (|) represents a phase boundary and the double vertical bar (||) represents the salt bridge.
The anode half cell is written on the left side of the salt bridge and the cathode half cell on the right side.
The anode and cathode are written on the extreme left and extreme right, respectively.
The emf of the cell is written on the right side after cell diagram.
Example
The net redox reaction of a galvanic cell is given below
2 Cr (s) + 3Cu2+ (aq) → 2Cr3+ (aq) + 3Cu (s)
Write the half reactions and describe the cell using cell notation.
Anodic oxidation 2Cr (s) → 2Cr3+ (aq) + 6e- .....(1)
Cathodic reduction : 3Cu2+ (aq) + 6e- → 3 Cu (s) .....(2)
Cell Notation is
Cr (s) | Cr3+ (aq) || Cu2+ (aq) | Cu(s)
Related Topics