Electro Chemistry - Thermodynamics of cell reactions | 12th Chemistry : UNIT 9 : Electro Chemistry
Chapter: 12th Chemistry : UNIT 9 : Electro Chemistry
Thermodynamics of cell reactions
Thermodynamics of cell reactions
We have just learnt that in a galvanic cell, the chemical energy is
converted into electrical energy. The electrical energy produced by the cell is
equal to the product of the total charge of electrons and the emf of the cell
which drives these electrons between the electrodes.
If ŌĆśnŌĆÖ is the number of moles of electrons exchanged between the
oxidising and reducing agent in the overall cell reaction, then the electrical
energy produced by the cell is given as below.
Electrical energy = Charge of ŌĆÖnŌĆÖ mole of electrons ├Ś Ecell ......(9.20)
Charge of 1 mole of electrons = one Faraday (1F)
Ōł┤ Charge of ŌĆÖnŌĆÖ mole of electrons = nF
Equation (9.20) ŌćÆ Electrical energy = nFEcell .......
(9.21)
Charge of one elctron = 1.602 ├Ś 10-19 C
Ōł┤ Charge one mole of elctron = 6.023 ├Ś 1023 ├Ś1.602 ├Ś10ŌłÆ19 C
= 96488 C
i.e., 1F 96500 C
This energy is used to do the electric work. Therefore the maximum work
that can be obtained from a galvanic cell is
(Wmax)cell = - nFEcell .....(9.22)
Here the (-) sign is introduced to indicate that the work is done by the
system on the surroundings.
We know from the Second Law of thermodynamics that the maximum work done
by the system is equal to the change in the Gibbs free energy of the system.
i.e., Wmax = ŌłåG .....(9.23)
From (9.22) and (9.23),
ŌłåG = - nFEcell
.....(9.24)
For a spontaneous cell reaction, the ŌłåG should be negative. The above expression (9.24)
indicates that Ecell should be positive to get a negative ŌłåG value.
When all the cell components are in their standard state, the equation
(9.24) becomes
ŌłåGo = - nFEcello
.....(9.25)
We know that the standard free energy change is related to the
equilibrium constant as per the following expression.
ŌłåGo = - RT
lnKeq .....(9.26)
Comparing (9.25) and (9.26),
nFEcell = RT lnKeq
ŌćÆ Ecell = [2.303 RT /nF] log Keq .....(9.27)
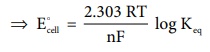
Related Topics