Chapter: Modern Analytical Chemistry: Titrimetric Methods of Analysis
Titrations in Nonaqueous Solvents
Titrations in Nonaqueous Solvents
Thus far we have assumed
that the acid and base are in an aqueous
solution. In- deed, water
is the most common solvent
in acid–base titrimetry. When considering the utility
of a titration, however, the
solvent’s influence cannot
be ignored.
The
dissociation, or autoprotolysis constant for a solvent, SH, relates the con-
centration of the
protonated solvent, SH2+, to that of the deprotonated solvent, S–. For amphoteric solvents, which can act as both proton
donors and proton
accep- tors, the autoprotolysis reaction is

You should recognize that Kw is just the specific form of Ks for water. The pH of a
solution is now seen to be a general statement about the relative
abundance of pro- tonated solvent
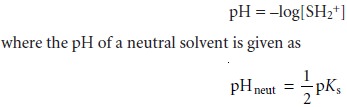
Perhaps the most obvious
limitation imposed by Ks is the change in pH during
a titration. To see why
this is so,
let’s consider the
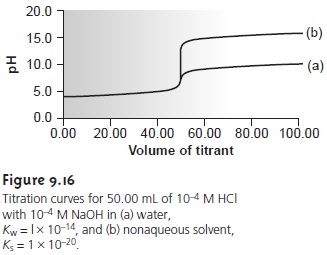
titration of a 50 mL solution of 10–4 M strong
acid with equimolar strong base. Before
the equivalence point,
the pH is determined by the untitrated strong acid, whereas
after the equivalence point the concentration of excess strong
base determines the pH. In an aqueous
solution the concentration of H3O+ when the titration is 90% complete is
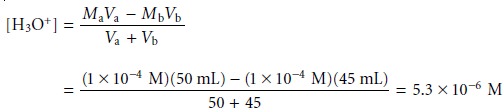
corresponding to a pH of 5.3. When the titration
is 110% complete, the concentra- tion of OH– is
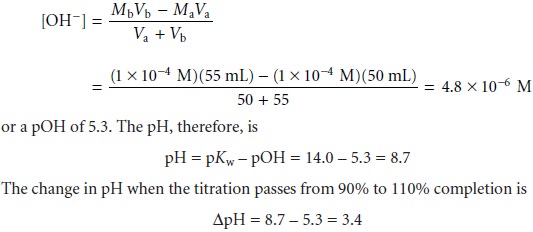
If the same titration is carried out in a nonaqueous solvent
with a Ks of 1.0 x 10–20, the pH when the titration is 90% complete is
still 5.3.
However, the pH when the titration
is 110% complete is now
pH = pKs – pOH = 20.0 –
5.3 = 14.7
In this case the change in pH of
∆pH = 14.7 – 5.3 = 9.4
is significantly greater than that
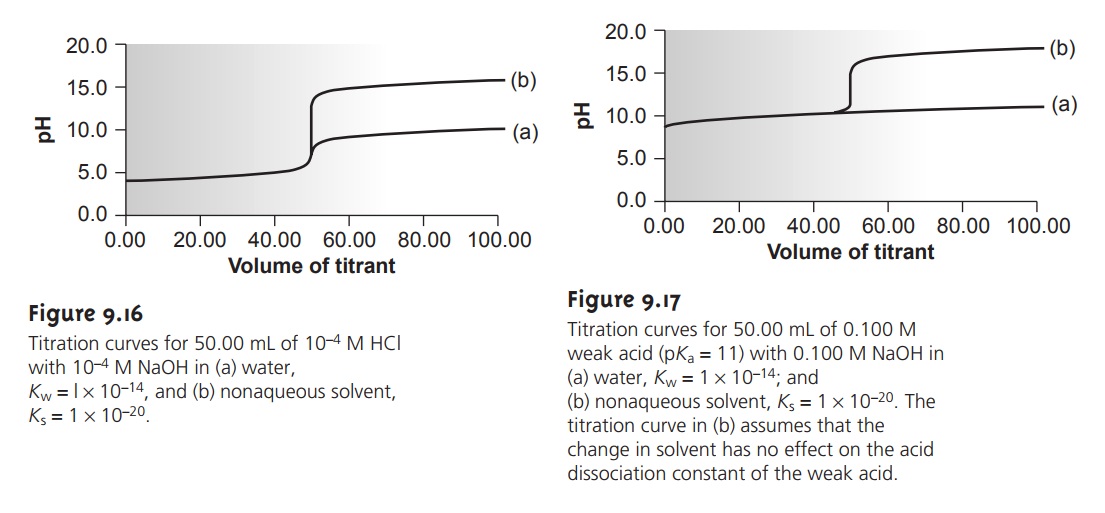
obtained when the titration is carried out in water. Figure 9.16 shows the
titration curves in both the aque- ous and nonaqueous solvents. Nonaqueous solvents also may be used
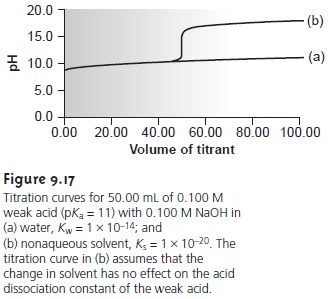
to increase the change in pH when titrating weak acids or bases (Figure 9.17).
Another parameter affecting the feasibility of a titration is the dis- sociation constant of the
acid or base
being titrated. Again,
the solvent plays an important role. In the Brønsted–Lowry view of acid–base
be- havior, the strength
of an acid or base is a relative measure
of the ease with which a proton is transferred from the acid to the solvent, or from
the solvent to the base.
For example, the strongest acid that can exist in water is H3O+. The acids HCl and HNO3 are considered
strong because they are better
proton donors than H3O+. Strong
acids essentially donate all their protons to H2O, “leveling” their acid strength to that of H3O+. In a different
solvent HCl and HNO3 may not
behave as strong
acids. When acetic acid, which is a weak acid, is placed in water, the
dis- sociation reaction
CH3COOH(aq)+ H2O(l) < == == > H3O+(aq)+ CH3COO–(aq)
does not proceed
to a significant extent because
acetate is a stronger base
than water and the hydronium ion is a stronger acid than acetic
acid. If acetic
acid is placed
in a solvent that is a stronger base than water,
such as ammonia,
then the reaction
CH3COOH + NH3 < == == > NH4+ + CH3COO–
proceeds to a greater extent.
In fact, HCl
and CH3COOH are
both strong acids
in ammonia.
All other things being equal, the strength
of a weak acid increases
if it is placed in a solvent that is more basic than water, whereas
the strength of a weak base in- creases if it is placed in a solvent
that is more acidic than water. In some cases,
how- ever, the opposite
effect is observed. For example, the pKb for ammonia is 4.76 in water
and 6.40 in the more acidic glacial
acetic acid. In contradiction to our expec- tations, ammonia is a weaker base
in the more
acidic solvent. A full description of the solvent’s effect
on a weak acid’s pKa or on the
pKb of a weak
base is beyond
the scope of this
text. You should
be aware, however, that titrations that
are not feasible in water may be feasible in a different solvent.
Related Topics