Chapter: Modern Analytical Chemistry: Titrimetric Methods of Analysis
Titrations Based on Acid–Base Reactions: Quantitative Applications
Quantitative Applications
Although many quantitative applications of acid–base titrimetry have been
replaced by other analytical methods, there are
several important applications that continue to be listed as standard methods. In this section
we review the
general application of acid–base titrimetry to the analysis
of inorganic and organic compounds, with an
emphasis on selected applications in environmental and clinical analysis. First, however, we discuss the selection and standardization of acidic and basic titrants.
Selecting and Standardizing a Titrant
Most common
acid–base titrants are not
readily available as primary standards and must be standardized before
they can be used in a quantitative analysis. Standardization is accomplished by titrating a known
amount of an appropriate acidic
or basic primary
standard.
The majority of titrations involving basic analytes, whether
conducted in aque- ous or nonaqueous solvents,
use HCl, HClO4, or H2SO4 as the titrant.
Solutions of these titrants are usually prepared by diluting a commercially available concentrated stock solution and are stable
for extended periods
of time. Since
the concentrations of concentrated acids are known only approximately,* the titrant’s concentration is determined by standardizing against one of the primary
standard weak bases
listed in Table 9.7.
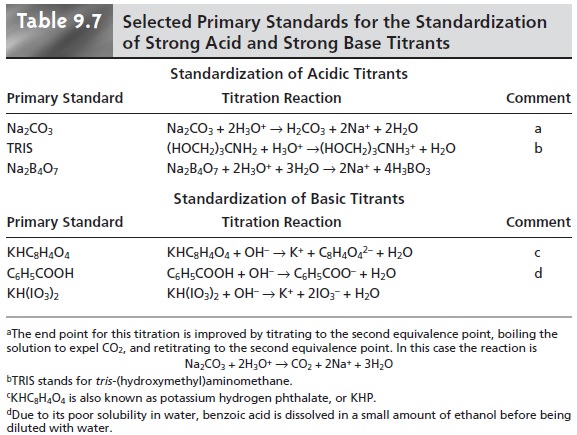
The
most common strong
base for titrating acidic analytes in aqueous solutions is NaOH. Sodium hydroxide is available both as a solid and as an approximately
50% w/v solution. Solutions of NaOH may
be standardized against
any of the
pri- mary weak acid standards listed in Table 9.7. The standardization of NaOH, how- ever, is complicated by potential contamination from the following reaction be- tween CO2 and OH–.
CO2(g) + 2OH–(aq) - > CO32–(aq) + H2O(l) ….
9.7
When CO2 is present, the volume of NaOH used in the titration is greater than that needed to neutralize the primary standard because some OH– reacts with the CO2
The calculated concentration of OH–, therefore, is too small.
This is not a problem when titrations involving NaOH are restricted to an end point pH less than 6.
Below this pH any CO32– produced in reaction 9.7 reacts with H3O+ to form car-
bonic acid.
CO32–(aq)+ 2H3O+(aq) → H2CO3(aq)+ 2H O(l)
Combining reactions 9.7 and 9.8 gives an overall reaction of
CO2(g)+ H2O(l) → H2CO3(aq)
which does not include OH–. Under these
conditions the presence
of CO2 does not
affect the quantity of OH– used in the titration and, therefore, is not a source of de-
terminate error.
|
3 |
CO32–(aq)+H3O+(aq) → HCO3–(aq)+H O(l)
and the net reaction between CO2 and OH– is
CO2(g)+ OH–(aq) → HCO3–(aq)
Under these conditions some OH– is consumed in neutralizing CO2. The result is a determinate error in the titrant’s concentration. If the titrant
is used to analyze an analyte that has the same end point pH as the primary standard
used during stan- dardization, the determinate errors
in the standardization and the analysis cancel, and accurate results may still be obtained.
Solid NaOH is always
contaminated with carbonate due to its contact with the
atmosphere and cannot be used to prepare
carbonate-free solutions of NaOH. So- lutions of carbonate-free NaOH can be prepared from 50% w/v NaOH since Na2CO3 is very insoluble in concentrated NaOH.
When CO2 is absorbed, Na2CO3 precipitates and settles to the bottom of the container, allowing access to the carbonate-free NaOH.
Dilution must be done with water that is free from dissolved CO2. Briefly
boiling the water
expels CO2 and, after cooling,
it may be used to pre-
pare carbonate-free solutions
of NaOH. Provided
that contact with the atmosphere is minimized, solutions of carbonate-free NaOH are relatively stable when stored in polyethylene bottles. Standard
solutions of sodium hydroxide should not be stored
in glass bottles
because NaOH reacts
with glass to form silicate.
Inorganic Analysis
Acid–base
titrimetry is a standard method for the quantitative analysis of many inorganic acids and bases.
Standard solutions of NaOH can be
used in the analysis of inorganic acids
such as H3PO4 or H3AsO4, whereas
standard solutions of HCl
can be used
for the analysis of inorganic bases
such as Na2CO3.
|
4 |
In this case, NH4+ can be converted to NH by neutralizing with strong base. The NH3, for which Kb is 1.8 x 10–5, is then removed by distillation and titrated with a standard strong acid titrant.
3NO3–(aq) + 8Al(s) + 5OH–(aq)+ 2H2O(l) → 8AlO2–(aq) + 3NH3 (aq)Inorganic analytes that are neutral
in aqueous solutions may still be analyzed if they
can be converted to an acid or base. For example, NO3– can be quantitatively
analyzed by reducing it to NH3 in a strongly
alkaline solution using Devarda’s alloy, a
mixture of 50% w/w Cu, 45% w/w Al, and 5% w/w Zn.
Acid–base titrimetry continues to be listed
as the standard method for the de- termination of alkalinity, acidity, and free CO2 in water and
wastewater analysis. Al-
kalinity is a measure of the acid-neutralizing capacity of a water sample
and is as- sumed to arise
principally from OH–, HCO3–, and CO32–, although
other weak bases, such
as phosphate, may
contribute to the
overall alkalinity. Total
alkalinity is determined by titrating with a standard
solution of HCl or H2SO4 to a fixed end
point at a pH of 4.5, or to the bromocresol green
end point. Alkalinity is reported as milligrams CaCO3 per liter.The NH3 is removed by distillation and
titrated with HCl.
Alternatively, NO3– can
be titrated as a weak
base in an acidic nonaqueous solvent such as anhydrous acetic acid, using HClO4 as a titrant.
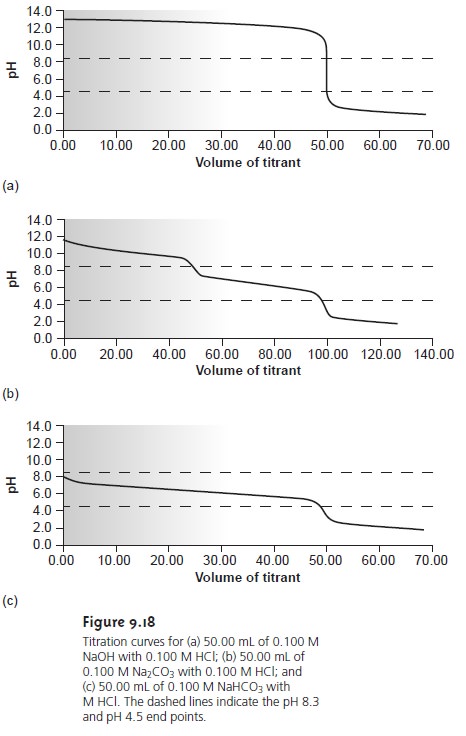
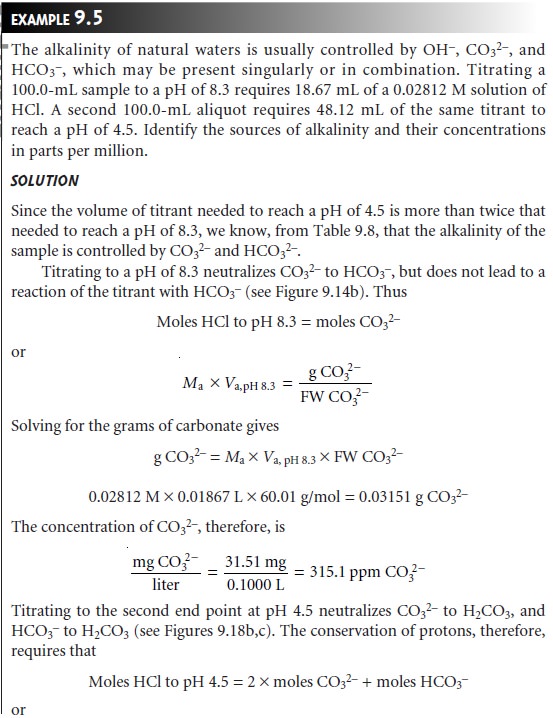
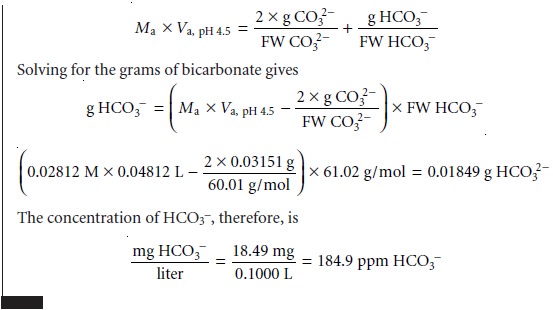
Mixtures of OH– and CO32–, or HCO3–
and CO32– alkalinities also are possible. Consider, for example, a mixture of OH– and CO32–. The volume of strong acid
needed to titrate OH– will be the same whether we titrate to the pH 8.3 or pH 4.5 end
point. Titrating CO32– to the end point
at a pH of 4.5,
however, requires twice as much strong acid as when titrating to the pH 8.3 end point. Consequently, when titrating a mixture
of these two ions, the volume of strong acid needed to reach the pH
4.5 end point
is less than twice that needed to reach the end point
at a pH of 8.3. For
a mixture of HCO3– and CO32–, similar reasoning shows that the volume of strong acid needed to reach the end point at a pH of 4.5 is more than twice that need
to reach the
pH 8.3 end
point. Solutions containing OH– and HCO3– alkalini- ties are
unstable with respect
to the formation of CO32– and do not exist.
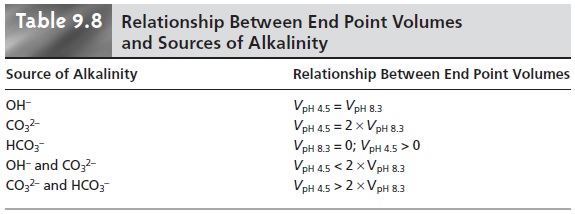
Table 9.8 summarizes the relationship between
the sources of alkalinity and the volume
of titrant needed to reach the
two end points.
Acidity is a measure of a water sample’s capacity for neutralizing base and is conveniently divided into strong acid and weak acid acidity. Strong acid acidity is due to the presence of inorganic acids, such as HCl, HNO3, and H2SO4, and is com- monly found in industrial effluents and acid mine drainage. Weak acid acidity is usually dominated by the formation of H2CO3 from dissolved CO2, but also includes contributions from hydrolyzable metal ions such as Fe3+, Al3+, and Mn2+. In addition, weak acid acidity may include a contribution from organic acids.
Acidity is determined by titrating with a standard
solution of NaOH to fixed end points at pH 3.7 and pH 8.3. These end points are located potentiometrically, using a pH meter, or by using an appropriate indicator
(bromophenol blue for pH
3.7, and metacresol purple or phenolphthalein for pH 8.3).
Titrating to a pH of 3.7 provides a measure of strong acid
acidity,* and titrating to a pH of 8.3
provides a measure of total acidity.
Weak acid acidity
is given indirectly as the difference be- tween the total
and strong acid acidities. Results
are expressed as the milligrams of CaCO3 per liter that could be neutralized by the water sample’s acidity.
An alterna- tive approach
for determining strong
and weak acidity
is to obtain a potentiometric titration curve
and use Gran plot methodology to determine the two equivalence points. This
approach has been
used, for example, in determining the
forms of acid- ity in atmospheric aerosols.
Water in contact with either the
atmosphere or carbonate-bearing sediments
contains dissolved or free CO2 that exists in equilibrium with gaseous CO2 and the aqueous carbonate species H2CO3, HCO3–, and CO32–. The
concentration of free CO2 is determined by titrating with a standard
solution of NaOH to the phenol-
phthalein end point,
or to a pH of 8.3, with
results reported as milligrams CO2 per liter. This analysis is essentially the
same as that
for the determination of total acid- ity, and can only
be applied to water samples
that do not
contain any strong
acid acidity.
Organic Analysis
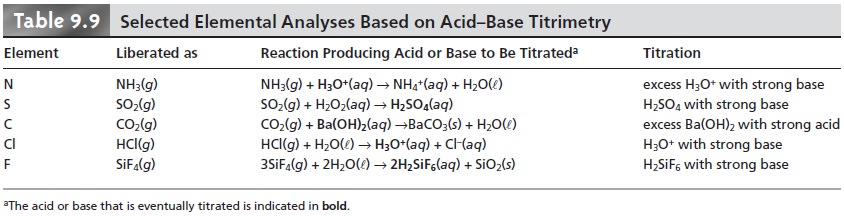
The use of acid–base titrimetry for the analysis of organic com- pounds continues to play an important role in pharmaceutical, biochemical, agri- cultural, and environmental laboratories. Perhaps the most widely employed acid–base titration is the Kjeldahl analysis for organic nitrogen, described earlier in Method 9.1. This method continues to be used in the analysis of caffeine and sac- charin in pharmaceutical products, as well as for the analysis of proteins, fertilizers, sludges, and sediments. Any nitrogen present in the –3 oxidation state is quantita- tively oxidized to NH4+. Some aromatic heterocyclic compounds, such as pyridine, are difficult to oxidize. A catalyst, such as HgO, is used to ensure that oxidation is complete. Nitrogen in an oxidation state other than –3, such as nitro- and azo- nitrogens, is often oxidized to N2, resulting in a negative determinate error. Adding a reducing agent, such as salicylic acid, reduces the nitrogen to a –3 oxidation state, eliminating this source of error. Other examples of elemental analyses based on the conversion of the element to an acid or base are outlined in Table 9.9.
Several organic functional groups have weak acid or weak base properties that allow their direct determination by an acid–base titration. Carboxylic (—COOH), sulfonic (—SO3H), and phenolic
(—C6H5OH) functional groups are weak acids
that can be successfully titrated
in either aqueous
or nonaqueous solvents. Sodium hydroxide is the titrant of choice for aqueous solutions. Nonaqueous titrations are often
carried out in a basic solvent, such as ethylenediamine, using tetrabutylam-
monium hydroxide, (C4H9)4NOH, as the titrant.
Aliphatic and aromatic
amines are weak bases
that can be titrated using
HCl in aqueous solution or HClO4 in glacial
acetic acid. Other functional groups
can be analyzed indirectly by use
of a func- tional group reaction that produces or consumes an acid or base. Examples are shown in Table 9.10.
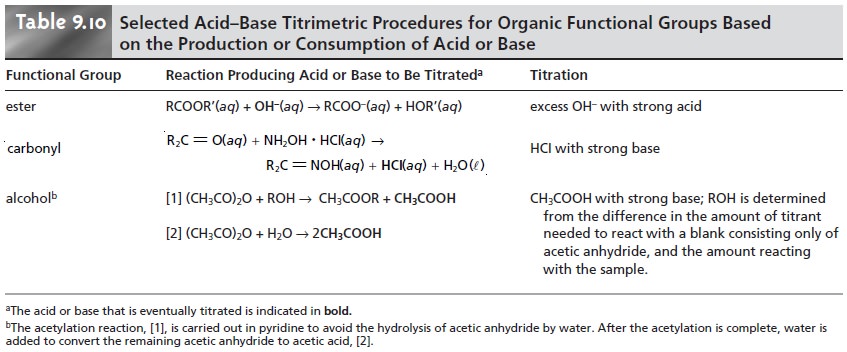
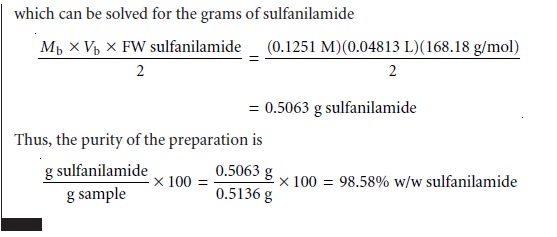
Many pharmaceutical compounds are
weak acids or bases that
can be analyzed by an aqueous or nonaqueous acid–base titration; examples include
salicylic acid, phenobarbital, caffeine, and sulfanilamide. Amino
acids and proteins
can be ana- lyzed in glacial
acetic acid, using
HClO4 as the titrant. For example, a procedure for determining the amount of nutritionally available protein has been developed that is
based on an acid–base titration of lysine residues.
Quantitative Calculations
In acid–base titrimetry the quantitative relationship be- tween the analyte and the titrant
is determined by the stoichiometry of the relevant reactions. As outlined, stoichiometric calculations may be simplified
by focusing on appropriate conservation principles. In an acid–base reaction
the number of protons
transferred between the acid and base is conserved; thus
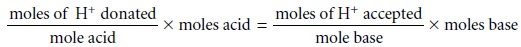
The following example demonstrates
the application of this approach in the direct analysis of a single analyte.
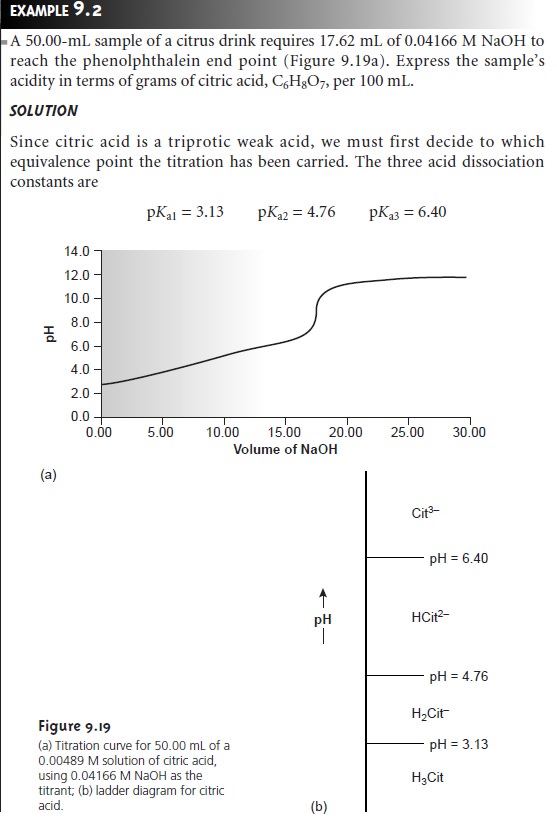

In
an indirect analysis
the analyte participates in one or more preliminary reac- tions that produce
or consume acid or base. Despite the additional complexity, the stoichiometry between the analyte and the amount
of acid or base produced
or con- sumed may be established by applying the conservation principles outlined in Sec- tion
2C. Example 9.3 illustrates the application of an indirect
analysis in which an
acid is produced.
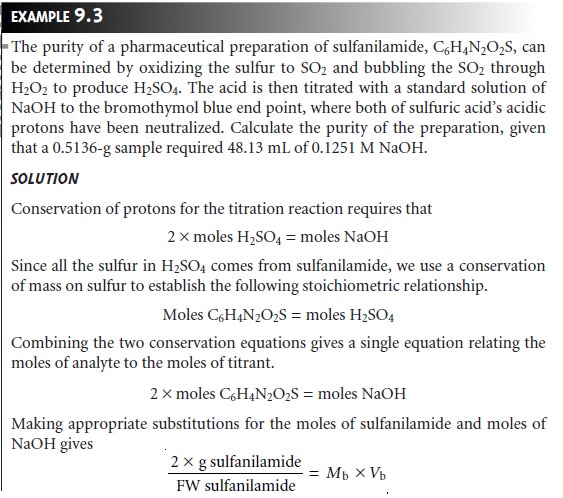

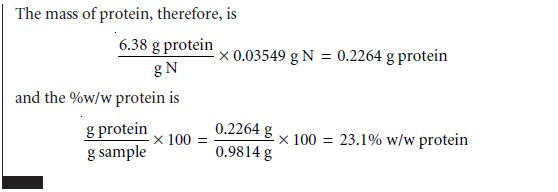
Earlier we noted that an acid–base titration may be used to analyze a mixture of acids
or bases by titrating to more than one equivalence point. The concentration of each analyte is determined by accounting for its contribution to the volume
of titrant needed to reach the
equivalence points.
Related Topics