Chapter: Modern Analytical Chemistry: Titrimetric Methods of Analysis
Quantitative Applications - Titrations Based on Redox Reactions
Quantitative Applications
As with acid–base and complexation titrations, redox titrations
are not frequently used in modern analytical laboratories. Nevertheless, several
important applications
continue to find favor in environmental, pharmaceutical, and industrial laborato- ries. In this section
we review the general application of redox titrimetry. We begin, however, with a brief discussion of selecting and characterizing redox titrants, and methods for controlling the
analyte’s oxidation state.
Adjusting the Analyte’s Oxidation State
If a redox
titration is to be used
in a quantitative analysis, the analyte must
initially be present
in a single oxidation state. For example, the iron
content of a sample can
be determined by a redox
titration in which Ce4+ oxidizes Fe2+ to Fe3+.
The process of preparing the sample for analysis
must
ensure that all iron is present as Fe2+. Depending on the sample and the method
of sample preparation, however, the iron may initially be present in both
the +2 and +3 oxidation
states. Before titrating, any Fe3+ that is present must be re- duced
to Fe2+. This type of pretreatment can be accomplished with an auxiliary re- ducing or oxidizing
agent.
Metals that are easily oxidized, such as Zn, Al, and Ag, can serve as auxiliary
re- ducing agents. The
metal, as a coiled wire or powder,
is placed directly
in the solu- tion where it reduces the analyte. Of course any unreacted auxiliary reducing agent will interfere with the analysis by reacting with
the titrant. The
residual auxiliary re- ducing agent, therefore, must be removed
once the analyte
is completely reduced. This can be accomplished by simply removing the coiled wire
or by filtering.
An alternative approach
to using an auxiliary reducing
agent is to immobilize it in a column.
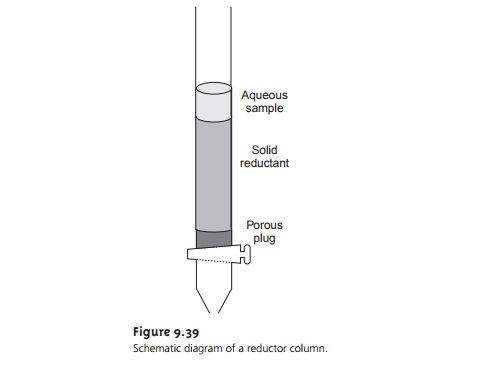
To prepare a reduction column,
an aqueous slurry of the finely divided metal is packed in a glass
tube equipped with
a porous plug
at the bottom
(Figure 9.39). The sample is placed at the top of the column and moves through
the column under the influence of gravity or vacuum suction.
The length of the reduction col- umn and the flow rate are selected
to ensure the analyte’s complete
reduction.
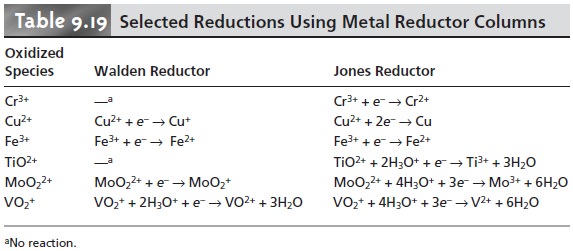
Two common reduction columns are used.
In the Jones reductor
the column is filled
with amalgamated Zn prepared by briefly placing
Zn granules in a solution of HgCl2 to form Zn(Hg). Oxidation of the amalgamated Zn
Zn(Hg)(s) < = = = = > Zn2+(aq) + Hg(l)+ 2e–
provides the electrons for reducing the analyte. In the Walden reductor the column is filled
with granular Ag metal. The solution containing the analyte is acidified with HCl
and passed through
the column where
the oxidation of Ag
Ag(s)+
Cl–(aq)<
== == > AgCl(s)+ e–
provides the necessary electrons for reducing
the analyte. Examples
of both reduc- tion columns are shown
in Table 9.19.
Several reagents are
commonly used as auxiliary
oxidizing agents, including ammonium peroxydisulfate, (NH4)2S2O8, and hydrogen peroxide, H2O2.
Ammo- nium peroxydisulfate is a powerful
oxidizing agent
S2O 2–(aq)+ 2e– < == == > 2SO42–(aq)
|
7 |
H2O2(aq)+ 2H3O+(aq)+ 2e– < = = = = > 4H2O(l)
provides another method for oxidizing
an analyte. Excess H2O2 also can be de-
stroyed by briefly boiling the solution.
Selecting and Standardizing a Titrant
In quantitative work the titrant’s concen- tration must remain
stable during the
analysis. Since titrants in a reduced
state are susceptible to air oxidation, most redox titrations are carried out using an oxidizing
agent as the titrant. The
choice of which
of several common
oxidizing titrants is best
for a particular analysis depends
on the ease
with which the
analyte can be oxidized.
Analytes that are
strong reducing agents
can be successfully titrated with a relatively
weak oxidizing titrant,
whereas a strong
oxidizing titrant is required for the analysis of analytes that are weak reducing
agents.
The two strongest oxidizing titrants are MnO4– and Ce4+, for which
the reduc- tion
half-reactions are
MnO4–(aq)+ 8H3O+(aq)+ 5e– t< = = = > Mn2+(aq) + 12H2O(l)
Ce4+(aq)+ e– <
== == > Ce3+(aq)
|
× |
Solutions of MnO4–
are prepared from KMnO4, which
is not available as a pri-
mary standard. Aqueous
solutions of permanganate are thermodynamically unsta- ble due to its ability to oxidize water.
4MnO4–(aq)+ 2H2O(l) < == == > 4MnO2(s)+ 3O2(g) + 4OH–(aq)
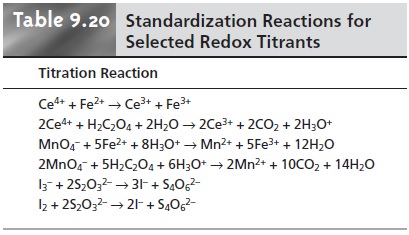
This reaction is catalyzed by the presence of MnO2, Mn2+, heat, light, and the pres- ence of acids and bases. Moderately stable solutions of permanganate can be pre- pared by boiling for an hour and filtering through a sintered glass filter to remove any solid MnO2 that precipitates. Solutions prepared in this fashion are stable for 1–2 weeks, although the standardization should be rechecked periodically. Stan- dardization may be accomplished using the same primary standard reducing agents that are used with Ce4+, using the pink color of MnO4– to signal the end point (Table 9.20).
Potassium dichromate is a relatively strong oxidizing agent
whose principal ad- vantages are its availability as a primary
standard and the long-term stability of its solutions. It is not, however, as strong an oxidizing agent
as MnO4– or Ce4+, which prevents its application to the analysis
of analytes that are weak reducing agents.
Its reduction half-reaction is
Cr2O72–(aq) + 14H3O+(aq)+ 6e– < = = = = > 2Cr3+(aq) + 21H2O(l)
Although solutions of Cr2O72– are orange and those
of Cr3+
are green, neither
color is intense enough
to serve as a useful
indicator. Diphenylamine sulfonic
acid, whose oxidized form is purple
and reduced form is colorless, gives a very distinct end point signal with Cr2O72–.
Iodine is another
commonly encountered oxidizing titrant. In comparison with MnO4–, Ce4+, and Cr2O72–, it is a weak oxidizing
agent and is useful only for the analysis of analytes that are strong
reducing agents. This apparent limitation, how- ever, makes I2 a more selective
titrant for the analysis of a strong reducing agent in
the presence of weaker reducing
agents. The reduction half-reaction for I2 is
I2(aq)+ 2e– < = = = = > 2I–(aq)
Because of iodine’s
poor solubility, solutions are prepared by adding an excess
of I–. The complexation reaction
I2(aq)+ I–(aq) < = = = = > I3–(aq)
increases the solubility of I2 by forming the more soluble
triiodide ion, I3–. Even
though iodine is present as I3– instead of I2, the
number of electrons in the reduc- tion half-reaction is unaffected.
I3–(aq)+ 2e– < = = = = > 3I–(aq)
Solutions of I3– are normally standardized against Na2S2O3
(see Table 9.20) using starch as a specific indicator for I3–.
Oxidizing titrants such as MnO4–, Ce4+, Cr2O72– and I3–, are used to titrate
ana- lytes that are
in a reduced state. When
the analyte is in an oxidized state,
it can be reduced with an auxiliary
reducing agent and titrated with an oxidizing
titrant. Al- ternatively, the analyte can be titrated
with a suitable reducing titrant.
Iodide is a relatively strong reducing agent
that potentially could
be used for the analysis
of an- alytes in higher oxidation states. Unfortunately, solutions of I– cannot be used as a
direct titrant because
they are subject
to the air
oxidation of I– to I3–.
3I–(aq)
< = = = = > I3–(aq)+ 2e–
Instead, an excess
of KI is added, reducing
the analyte and liberating a stoichiometric amount
of I3–. The
amount of I3– produced is then determined by a back
titra- tion using Na2S2O3 as a reducing titrant.
2S2O 2–(aq) < = = = = > S4O 2–(aq)+ 2e–
|
3 |
IO3–(aq)+ 8I–(aq)+ 6H3O+(aq) <
== = > 3I3–(aq)+ 9H O(l)
|
3 3 |
|
× |
|
7 |
Inorganic Analysis
Redox titrimetry has been used for the analysis of a wide range
of inorganic analytes. Although many of these methods
have been replaced by newer methods,
a few continue to be listed as standard methods
of analysis. In this
section we consider the application of redox titrimetry to several important envi- ronmental, public health,
and industrial analyses.
One of the most important
applications of redox titrimetry is in evaluating the chlorination of public water supplies. In Method 9.3 an
approach for determining the total chlorine residual
was described in which the oxidizing power of chlorine
is used to oxidize
I– to I3–. The amount
of I3–
formed is determined by a back
titration with S2O32–.
The efficiency of chlorination depends on the form of the chlorinating
species. For this reason it is important
to distinguish between
the free chlorine residual, due to Cl2, HOCl, and OCl–, and the combined chlorine
residual. The latter
form of chlorine
results from the reaction of ammonia with the free chlo-
rine residual, forming
NH2Cl, NHCl2, and
NCl3. When a sample of iodide-free
chlorinated water is mixed with an excess of the indicator N,N-diethyl-p- phenylenediamine (DPD),
the free chlorine
oxidizes a stoichiometric portion of DPD to its red-colored form. The oxidized
DPD is then titrated back to its color- less form with ferrous
ammonium sulfate, with the volume
of titrant being
pro- portional to the amount of free residual
chlorine. Adding a small amount of KI reduces monochloramine, NH2Cl, forming
I3–. The I3– then oxidizes
a portion of the
DPD to its
red-colored form. Titrating the oxidized DPD
with ferrous am- monium sulfate
yields the amount of NH2Cl in the sample. The amount of dichloramine and trichloramine are determined in a similar
fashion.
The methods described earlier for determining the total, free,
or combined chlorine residual
also are used in establishing the chlorine demand of a water sup- ply. The chlorine demand is defined as the quantity of chlorine that must be added to a water
supply to completely react with any substance that can be oxi-
dized by chlorine while also
maintaining the desired
chlorine residual. It is deter- mined by adding progressively greater amounts of chlorine to a set of samples drawn from the water
supply and determining the total, free,
or combined chlo- rine residual.
Another important example
of redox titrimetry that finds applications in both public health
and environmental analyses is the determination of dissolved oxygen. In natural waters the level of dissolved O2 is important for two reasons:
it is the most readily available oxidant for the biological oxidation of inorganic and organic
pollutants; and it is necessary for the support
of aquatic life.
In wastewater treat- ment plants, the control
of dissolved O2 is essential for the aerobic
oxidation of waste materials. If the level
of dissolved O2 falls below a critical value,
aerobic bacte- ria are replaced by anaerobic bacteria, and the oxidation of organic waste
produces undesirable gases such as CH4 and H2S.
One standard method for determining the dissolved O2 content of natural
wa- ters and wastewaters is the Winkler
method. A sample
of water is collected in a fash- ion that prevents its
exposure to the
atmosphere (which might
change the level
of dissolved O2). The sample is then treated
with a solution of MnSO4, and then with a
solution of NaOH and KI. Under these
alkaline conditions Mn2+ is oxidized
to MnO2 by the dissolved oxygen.
2Mn2+(aq) + 4OH–(aq)+ O2(aq) → 2MnO2(s)+ 2H2O(l)
After the reaction
is complete, the solution is acidified with H2SO4. Under
the now acidic conditions I– is oxidized to I3– by MnO2.
|
3 |
The amount of I3– formed is determined by titrating with S2O32– using starch as an indicator. The Winkler method is
subject to a variety of interferences, and
several modifications to the original procedure have been proposed. For example, NO2– in-
terferes because it can reduce
I3– to I– under acidic
conditions. This interference is eliminated by adding
sodium azide, NaN3, reducing NO2– to N2. Other reducing agents, such as Fe2+,
are eliminated by pretreating the sample with KMnO4, and de-
stroying the excess permanganate with K2C2O4.
|
× × |
py . I2 + py . SO2 + py+ H2O → 2py . HI+
py . SO3
Methanol is included to prevent the
further reaction of py . SO3 with
water. The titration’s end point is signaled when the solution
changes from the yellow color of
the products to the brown color of the Karl Fischer reagent.
Organic Analysis
Redox titrimetric methods
also are used
for the analysis of or- ganic analytes. One important example
is the determination of the chemical oxygen demand (COD) in natural
waters and wastewaters. The COD provides
a measure of the
quantity of oxygen
necessary to completely oxidize all the organic matter
in a sample to CO2 and H2O. No attempt is made to correct for organic matter
that can- not be decomposed biologically or for which
the decomposition kinetics are very slow. Thus,
the COD always
overestimates a sample’s
true oxygen demand.
The de- termination of COD is
particularly important in managing industrial wastewater treatment facilities where it is used to monitor the
release of organic-rich wastes into municipal sewer
systems or the environment.
The COD is determined
by refluxing the sample in the presence of excess K2Cr2O7, which
serves as the oxidizing agent.
The solution is acidified with H2SO4,
and Ag2SO4 is added as a catalyst to speed the
oxidation of low-molecular-weight fatty acids.
Mercuric sulfate, HgSO4, is added to complex any chloride that is pres- ent, thus preventing the precipitation of the Ag+ catalyst as AgCl. Under
these con- ditions, the
efficiency for oxidizing organic matter is 95–100%. After
refluxing for 2h, the solution is cooled to room temperature, and the excess
Cr2O72– is deter- mined by a back titration, using ferrous ammonium
sulfate as the titrant and fer-
roin as the indicator. Since
it is difficult to completely remove all traces
of organic matter from the reagents, a blank titration must be performed. The difference in the
amount of ferrous ammonium sulfate
needed to titrate
the blank and the sample is
proportional to the COD.
Iodine has been used as an oxidizing titrant
for a number of compounds
of pharmaceutical interest. Earlier
we noted that
the reaction of S2O32– with I3– pro-
duces the tetrathionate ion, S4O62–.
The tetrathionate ion is actually
a dimer consist- ing of two thiosulfate ions connected through
a disulfide (-S-S-)
linkage. In the same
fashion, I3– can be used to titrate mercaptans of the general
formula RSH, forming the dimer RSSR as a product. The amino acid cysteine also can be titrated
with I3–. The product of this titration is cystine, which
is a dimer of cysteine. Triio- dide also can be used for the analysis of ascorbic acid (vitamin C) by oxidizing the enediol functional group to an alpha diketone
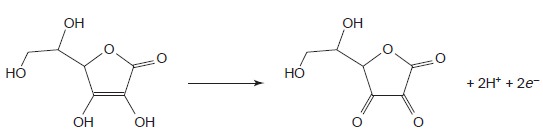
and
for the analysis
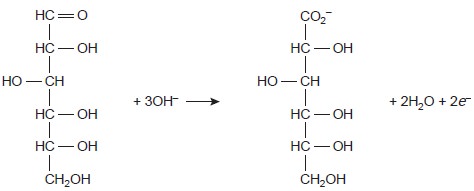
of reducing sugars,
such as glucose,
by oxidizing the aldehyde
functional group to a carboxylate ion in a basic solution.
Organic compounds containing a hydroxyl, carbonyl, or amine functional group adjacent to a hydoxyl or carbonyl group
can be oxidized using metaperio- date, IO3–, as an oxidizing titrant.
IO3–(aq)+H
O(l)+ 2e– → IO3–(aq) + 2OH–(aq)
|
4 |
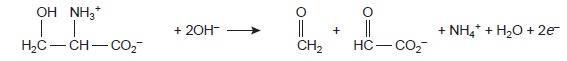
The analysis is conducted by adding a known excess
of IO3– to the solution contain- ing the analyte and allowing the oxidation to take place
for approximately 1 h at room
temperature. When the oxidation is complete, an excess of KI is added, which reacts with the unreacted IO3– to form
IO3– and I3–.
IO3–(aq)+
3I–(aq)+H O(l) → IO3–(aq)+I3–(aq) + 2OH–(aq)
he I3– is then determined by titrating with S2O32– using
starch as an indicator.
Quantitative Calculations
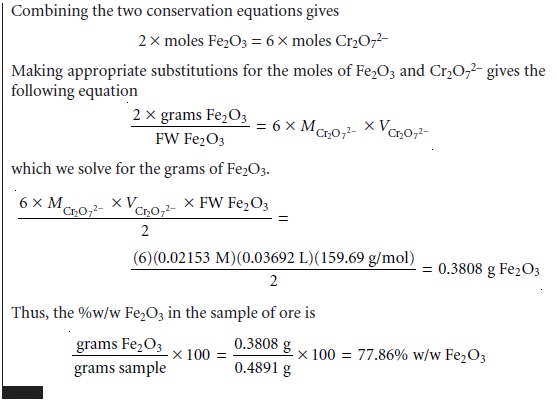
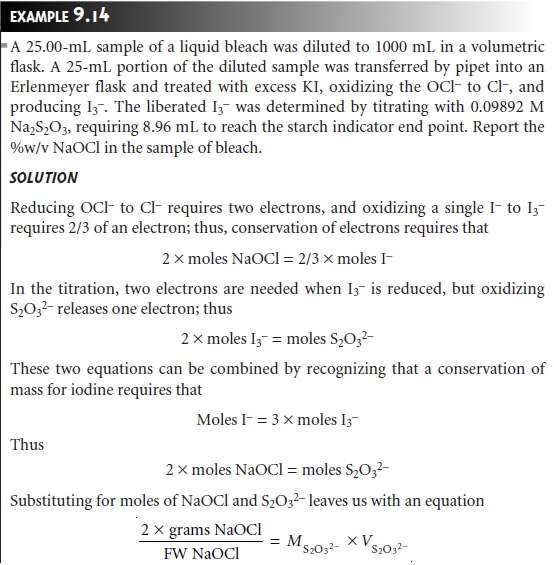
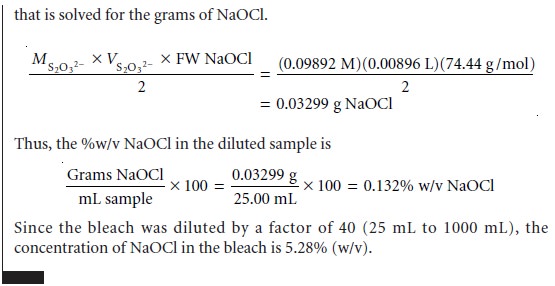
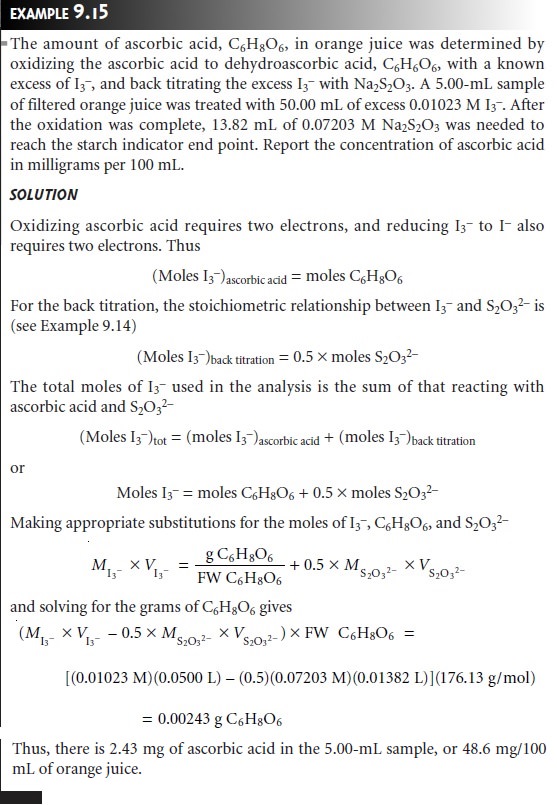
The stoichiometry of a redox reaction is given by the con- servation of electrons
between the oxidizing
and reducing agents;
thus

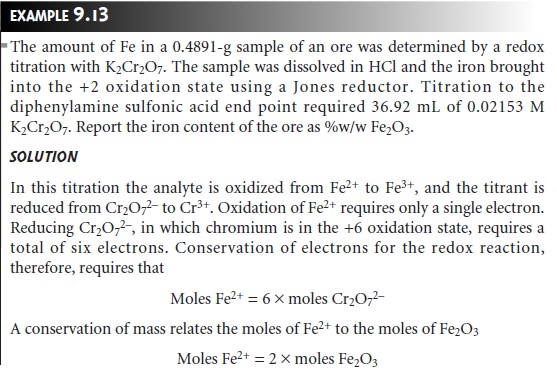
Example 9.13 shows how
this equation is applied to an analysis based on a direct
titration.
Related Topics