Chapter: Modern Analytical Chemistry: Titrimetric Methods of Analysis
Quantitative Applications - Titrations Based on Complexation Reactions
Quantitative Applications
With a few exceptions, most quantitative applications of
complexation titrimetry have been replaced
by other analytical methods. In this section we review the gen-
eral application of complexation titrimetry with an emphasis on selected
applica- tions from the analysis of water and wastewater. We begin, however,
with a discus- sion of the
selection and standardization of complexation titrants.
Selection and Standardization of Titrants
EDTA is a versatile titrant that can be
used for the analysis of virtually all
metal ions. Although EDTA is the
most com- monly employed
titrant for complexation titrations involving metal ions, it cannot be used for the direct analysis
of anions or neutral ligands.
In the latter case, stan- dard solutions of Ag+ or Hg2+ are used as the titrant.
Solutions
of
EDTA
are
prepared
from
the
soluble
disodium
salt, Na2H2Y•
2H2O. Concentrations can be determined directly from
the known mass of EDTA; however,
for more accurate
work, standardization is accomplished by titrating against a solution
made from the primary standard
CaCO3. Solutions of Ag+ and Hg2+ are prepared from AgNO3 and Hg(NO3)2, both
of which are
sec- ondary standards. Standardization is accomplished by titrating against
a solution prepared from primary standard
grade NaCl.
Inorganic Analysis
Complexation
titrimetry continues to be listed
as a standard method for the determination of hardness, Ca2+,
CN–, and Cl– in water and waste- water analysis. The evaluation of hardness was
described earlier in Method 9.2.
The determination of Ca2+ is complicated by the presence of Mg2+, which
also reacts with EDTA. To prevent
an interference from Mg2+, the pH is adjusted to 12–13,
precipitating any Mg2+ as Mg(OH)2. Titrating with EDTA using
murexide or Eri- ochrome Blue Black R as a visual indicator gives the concentration of Ca2+.
Cyanide is determined at concentrations greater
than 1 ppm by making
the sample alkaline with NaOH and titrating
with a standard
solution of AgNO3,
forming the soluble Ag(CN)2– complex. The end point is determined using p-dimethylamino benzalrhodamine as a visual
indicator, with the solution turn- ing from yellow to a salmon
color in the presence of excess Ag+.
Chloride is determined by titrating with Hg(NO3)2, forming
soluble HgCl2. The sample
is acidified to within the pH range
of 2.3–3.8 where
diphenylcarbazone, which forms a colored complex
with excess Hg2+, serves as the visual indicator. Xy- lene
cyanol FF is added as a pH indicator to ensure that the pH is within
the desired range. The initial solution
is a greenish blue, and the titration is carried out to a purple end point.
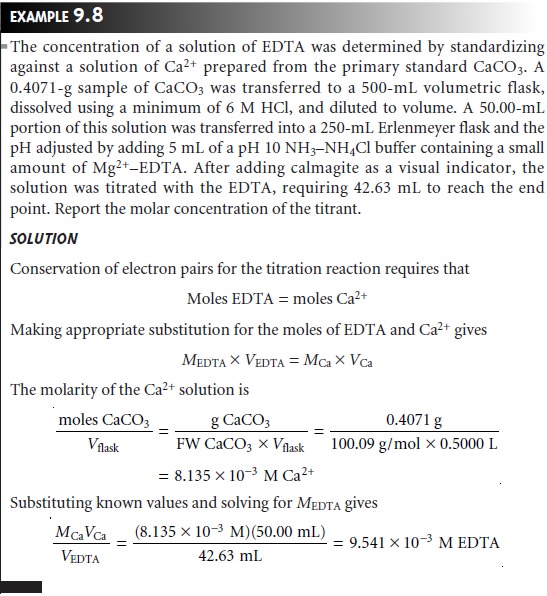
Quantitative Calculations
The stoichiometry
of complexation reactions is given by the conservation of electron pairs
between the ligand,
which is an electron-pair
donor, and the metal, which
is an electron-pair acceptor; thus

This is simplified for titrations involving EDTA where the stoichiometry is always
1:1 regardless of how many electron pairs are involved
in the formation of the metal–ligand complex.
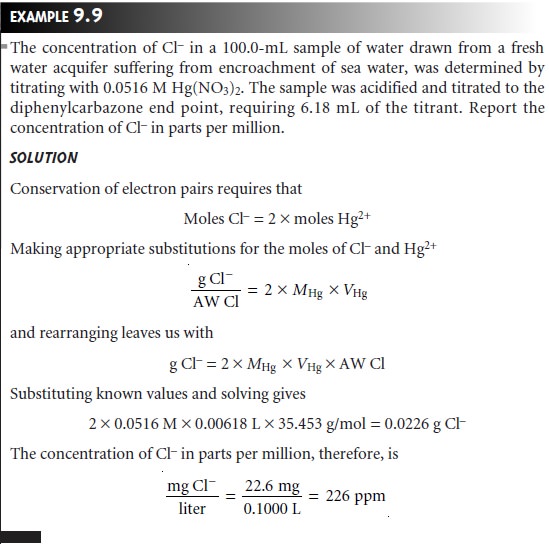
The
principle of the conservation of electron pairs
is easily extended
to other com- plexation reactions, as shown
in the following example.
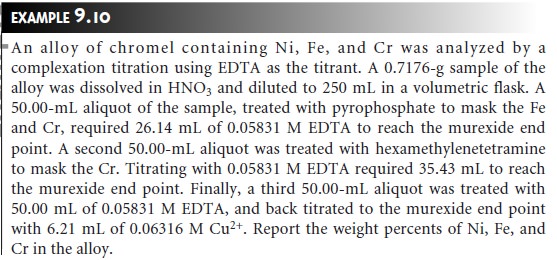
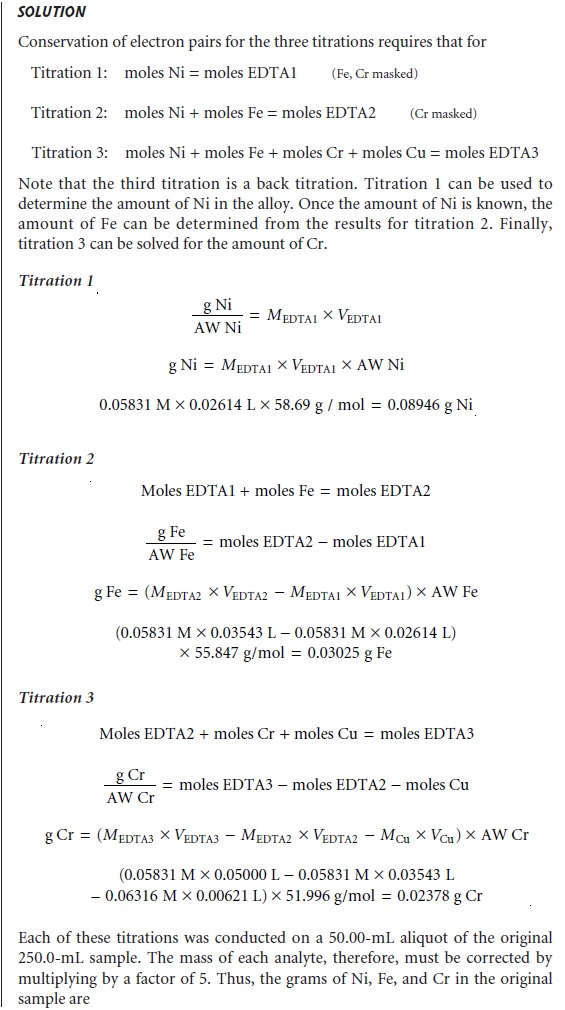
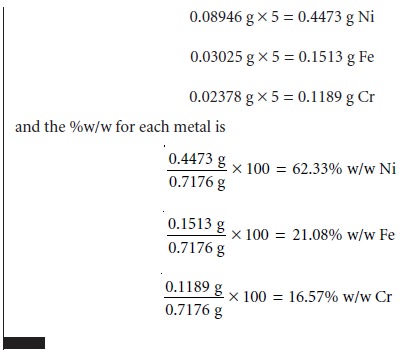
Related Topics