Chapter: Modern Analytical Chemistry: Titrimetric Methods of Analysis
Chemistry and Properties of EDTA
Chemistry and Properties of EDTA
Ethylenediaminetetraacetic acid, or EDTA,
is
an aminocar-
boxylic acid. The
structure of EDTA
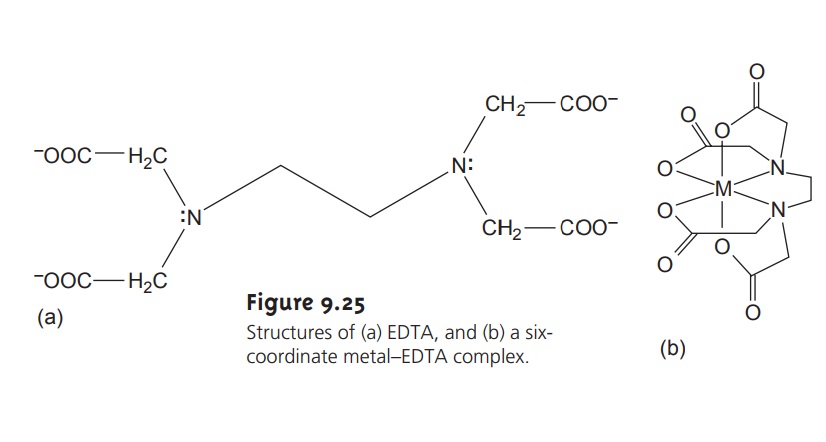
is shown in Figure 9.25a. EDTA, which is a Lewis acid, has six binding sites (the four car-
boxylate groups and the two amino groups),
providing six pairsof
electrons. The resulting metal–ligand complex, in which EDTA forms a cage-like structure around the metal ion (Figure 9.25b),
is very stable. The actual number of coordination sites depends on the size of the metal ion; however, all metal–EDTA
complexes have a 1:1 stoichiometry.
Metal–EDTA Formation Constants
To
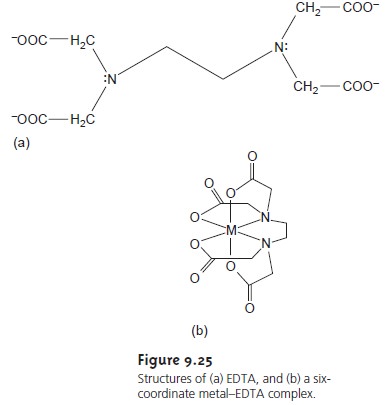
illustrate the formation of a metal–EDTA complex consider the reaction between
Cd2+ and EDTA
Cd2+(aq)+ Y4–(aq) t<
== è CdY2–(aq)
where Y4– is a shorthand notation
for the chemical
form of EDTA shown in Figure
9.25. The formation constant for this reaction
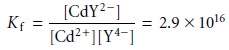 9.11
9.11
is quite large,
suggesting that the reaction’s equilibrium position lies far to the right.
Formation constants for other metal–EDTA complexes are found in Appendix
3C.
EDTA Is a Weak Acid
Besides its
properties as a ligand, EDTA
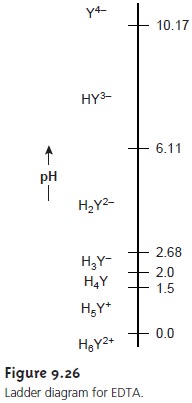
is also a weak acid. The fully protonated form of EDTA,
H6Y2+, is a hexaprotic weak acid with succes-
sive pKa values of
pKa1 = 0.0
pKa2 = 1.5 pKa3 = 2.0 pKa4 = 2.68 pKa5 = 6.11 pKa6 = 10.17
The first four values are for the carboxyl protons,
and the remaining two values are for
the ammonium protons.
A ladder diagram
for EDTA is shown in Figure 9.26. The species Y4– becomes the predominate form
of EDTA at pH levels
greater than 10.17. It is only for
pH levels greater
than 12 that
Y4– becomes the only significant form of EDTA.
Conditional Metal–Ligand Formation Constants
Recognizing EDTA’s acid–base properties is important. The formation constant
for CdY2– in
equation 9.11 as- sumes that EDTA is present as Y4–. If we restrict
the pH to levels greater
than 12, then equation
9.11 provides an adequate description of the formation of CdY2–. For pH
levels less than
12, however, Kf overestimates the stability of the CdY2– complex.
At any pH a mass
balance requires that
the total concentration of unbound EDTA equal
the combined concentrations of each of its forms.
CEDTA = [H6Y2+]+ [H5Y+]+ [H4Y]+
[H3Y–]+
[H2Y2–]
+ [HY3–]+
[Y4–]
To
correct the formation constant for EDTA’s
acid–base properties, we must ac count for the
fraction, αY4–, of EDTA present as Y4–.
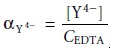 9.12
9.12
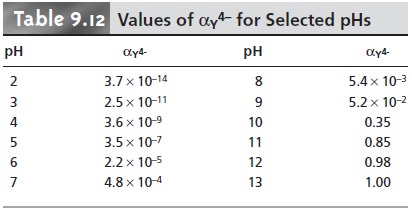
Values of αY4– are shown
in Table 9.12. Solving equation 9.12 for [Y4–] and substi- tuting into the
equation for the formation constant gives
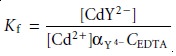
If we fix the pH using a buffer, then αY4– is a constant. Combining αY4– with Kf
gives
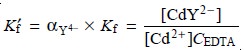 9.13
9.13
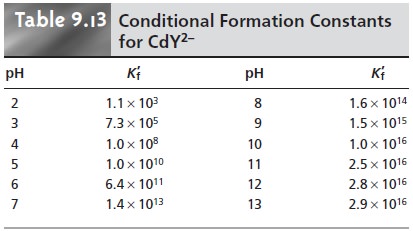
where Kf´ is a conditional formation constant whose value depends on the pH. As
shown in Table 9.13 for CdY2–, the conditional formation constant becomes
smaller, and the complex becomes
less stable at lower pH levels.
EDTA Must Compete with Other Ligands
To maintain a constant pH,
we must add a
buffering agent. If one of the buffer’s
components forms a metal–ligand complex with Cd2+, then EDTA must compete with the ligand for Cd2+. For example,
an NH4+/NH3 buffer includes
the ligand NH3, which forms several
stable Cd2+–NH3
complexes. EDTA forms
a stronger complex
with Cd2+ and will displace NH3. The presence of NH3, however, decreases the stability of the Cd2+–EDTA complex.
We can account
for the effect
of an auxiliary complexing agent, such as NH3,
in the same way we accounted for the effect
of pH. Before adding EDTA,
a mass balance on Cd2+ requires that the total
concentration of Cd2+,
CCd, be
CCd = [Cd2+] + [Cd(NH3)2+] + [Cd(NH3) 2+] + [Cd(NH3) 2+] + [Cd(NH3) 2+]
The fraction, αCd2+, present as
uncomplexed Cd2+
is
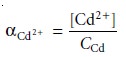 9.14
9.14
Solving equation 9.14 for
[Cd2+] and
substituting into equation 9.13 gives
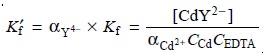
If
the concentration of NH3 is held constant, as it usually
is when using
a buffer, then we can rewrite
this equation as
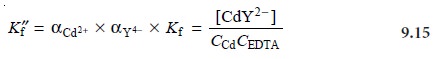
where Kf˝ is a new conditional formation constant accounting for both pH and the presence of an auxiliary
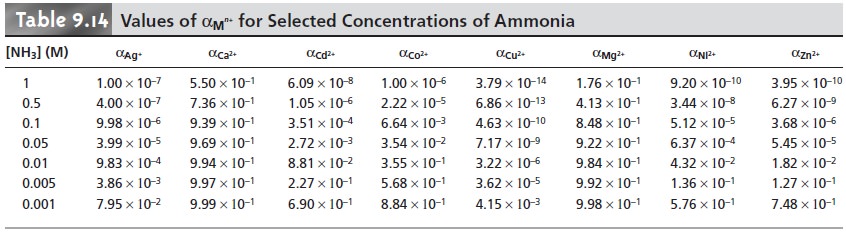
complexing agent. Values of αMn+ for several
metal ions are provided in Table 9.14.
Related Topics