Chapter: Modern Analytical Chemistry: Titrimetric Methods of Analysis
Quantitative Applications - Precipitation Titration
Quantitative Applications
Precipitation titrimetry is rarely
listed as a standard method
of analysis, but may
still be useful as a secondary analytical method for verifying results obtained by other
methods. Most precipitation titrations involve Ag+ as either an analyte
or
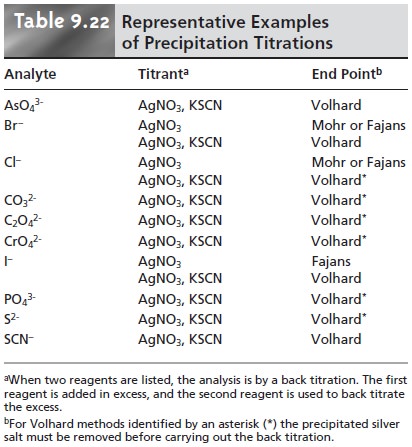
Those titrations in which Ag+ is the titrant are called argentometric titra- tions. Table 9.22 provides a list of several typical
precipitation titrations.
Quantitative Calculations
The stoichiometry of a precipitation reaction is given by the conservation of charge between
the titrant and analyte; thus
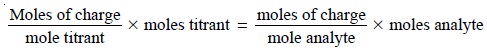
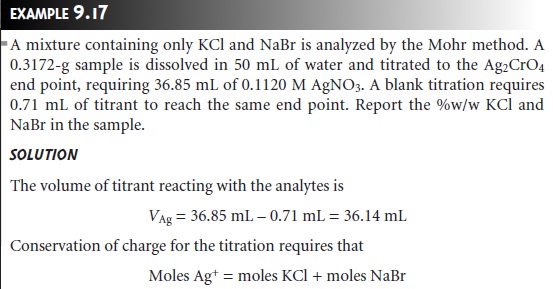
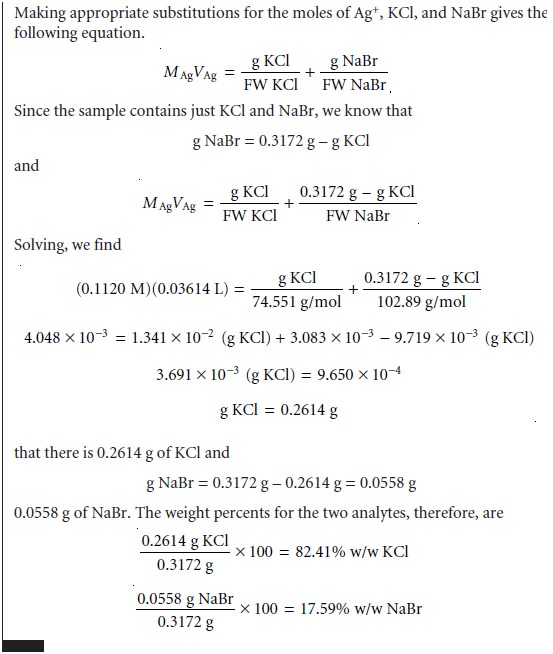
Example 9.17 shows how
this equation is applied to an analysis based on a direct
titration.
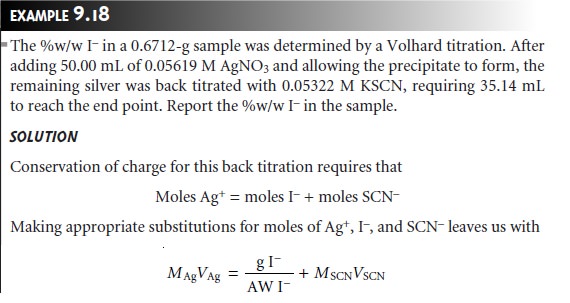
The
analysis for I– using the Volhard method
requires a back titration. A typical
calculation is shown in the following example.
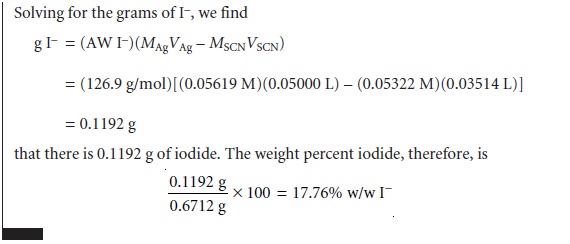
Related Topics