Chapter: Modern Analytical Chemistry: Titrimetric Methods of Analysis
Evaluation of Acid–Base Titrimetry
Evaluation of Acid–Base Titrimetry
Scale of Operation
In
an acid–base titration the volume of titrant needed
to reach the equivalence point is proportional to the absolute
amount of analyte present in the analytical solution. Nevertheless, the
change in pH at the
equiv- alence point, and thus the utility of an acid–base titration, is a function of the
analyte’s concentration in the solution
being titrated.
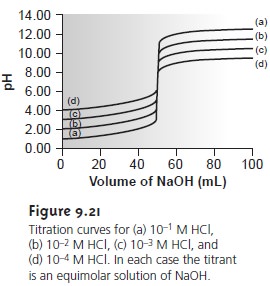
When the sample is available as a solution, the smallest concentration of analyte that can be readily
analyzed is approximately 10–3 M
(Figure 9.21). If, for
example, the analyte
has a gram formula weight
of 120 g/mol, then the lower concentration limit is 120 ppm. When the analyte is a solid,
it must first be
placed into solution, the volume of which must
be sufficient to allow the titration’s end point to be monitored using a visual
indicator or a suitable
probe. If we assume a minimum volume
of 25 mL, and a lower concentration limit of 120 ppm,
then a sample
containing at least
3 mg of analyte is re-
quired. Acid–base titrations involving solid or solution samples,
therefore, are generally limited
to major and
minor analytes. The analysis
of gases can be extended
to trace analytes
by pulling a large vol- ume
of the gas
through a suitable collection solution.
Efforts have been
made to develop
methods for conducting acid–base titrations on a much smaller
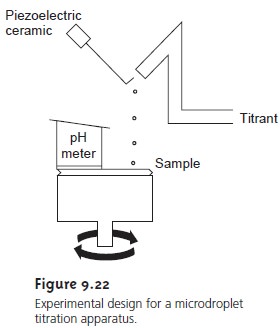
scale. In one
experimental design, samples
of 20–100 μL were
held by capillary action between a flat-surface pH electrode
and a stainless steel rod. The titrant
was added by using the oscillations of a piezoelectric ceramic
device to move an angled
glass rod in and out of atube
connected to a reservoir containing the titrant (see
Figure 9.22). Each time the glass tube
was withdrawn an approximately 2-nL
microdroplet of titrant was released. The microdroplets were allowed to fall onto the steel rod containing the sample,
with mixing accomplished by spinning the rod
at
120 rpm. A total of 450 microdroplets, with a combined
volume of 0.81–0.84 μL, was dispensed
between each pH measurement. In this fashion a
titration curve was constructed. This method was used to titrate solutions of 0.1 M HCl and 0.1 M CH3COOH with 0.1 M NaOH. Absolute errors ranged from
a minimum of +0.1% to a maximum
of –4.1%, with
relative standard deviations from 0.15% to 4.7%. The smallest volume
of sample that was
successfully titrated was 20 μL.
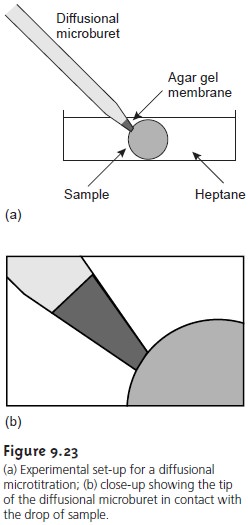
More recently, a method has been described in which the acid–base titration is conducted within a single drop of solution.11 The titrant
is added using
a microbu- ret fashioned from a glass
capillary micropipet (Figure
9.23). The microburet has a 1–2 μm tip filled
with an agar
gel membrane. The
tip of the
microburet is placed within a drop of the sample
solution, which is suspended in heptane, and
the titrant is allowed
to diffuse into the sample.
The titration is followed visually
using a col- ored indicator, and the time needed
to reach the end point
is measured. The rate of the
titrant’s diffusion from the microburet must be determined by calibration. Once calibrated, the end point time can be converted to an end point volume.
Sam- ples usually consisted
of picoliter volumes
(10–12 L), with the smallest
sample being 0.7 pL. The precision of the titrations was usually about
2%.
Titrations conducted with microliter or picoliter sample volumes require
a smaller absolute amount of analyte.
For example, diffusional titrations have been successfully conducted on as little as 29 femtomoles (10–15 mol) of nitric acid.
Nev- ertheless, the analyte
must still be present in the sample
at a major or minor
level for the titration to be performed accurately and precisely.
Accuracy
When working with macro–major and macro–minor samples,
acid–base titrations can
be accomplished with
relative errors of 0.1–0.2%. The
prin- cipal limitation to accuracy is the difference between the end point and the equiva- lence point.
Precision
The relative
precision of an acid–base titration
depends primarily on the
precision with which
the end point
volume can be measured and the precision of the end point
signal. Under optimum
conditions, an acid–base titration can be ac-
complished with a relative precision of 0.1–0.2%. The relative precision can be im- proved by using the largest volume
buret that is feasible and ensuring that most of its
capacity is used
to reach the
end point. Smaller
volume burets are
used when the cost of reagents or waste disposal
is of concern or when the titration must be com- pleted quickly to avoid competing chemical
reactions. Automatic titrators
are par- ticularly useful for titrations requiring small volumes
of titrant, since the precision with which the volume can be measured is significantly better (typically about 100.05%
of the buret’s volume).
The
precision of the end point
signal depends on the method
used to locate
the end point and the shape of the titration curve. With a visual indicator, the precision of the end point
signal is usually
between 0.03 mL and 0.10 mL. End points deter- mined by direct monitoring often can be determined with a greater
precision.
Sensitivity
For an acid–base titration
we can write the following
general analytical equation
Volume of titrant = k x moles of analyte
where k, the sensitivity, is determined by the stoichiometric relationship between
analyte and titrant. Note that this equation
assumes that a blank has been analyzed to correct the signal
for the volume
of titrant reacting
with the reagents.
Consider, for example, the determination of sulfurous acid,
H2SO3, by titrating
with NaOH to the first
equivalence point. Using
the conservation of protons, we write
Moles NaOH = moles H2SO3
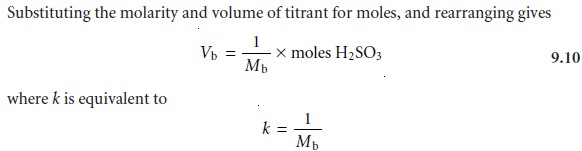
There are two ways in which
the sensitivity can be increased. The first, and most ob- vious, is to decrease
the concentration of the titrant,
since it is inversely propor- tional to the sensitivity, k. The second method,
which only applies
if the analyte is
multiprotic, is to titrate to a later
equivalence point. When H2SO3 is titrated to the
second equivalence point,
for instance, equation
9.10 becomes
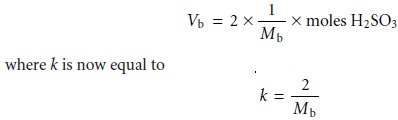
In practice, however, any improvement in the sensitivity of an
acid–base titration due to an increase in k is offset
by a decrease in the
precision of the
equivalence point volume when the buret
needs to be refilled. Consequently, standard analytical
procedures for acid–base titrimetry are usually written
to ensure that titrations re- quire
60–100% of the buret’s volume.
Selectivity
Acid–base titrants
are not selective. A strong base titrant,
for example, will neutralize any acid, regardless of strength. Selectivity, therefore, is determined by the relative acid
or base strengths of the analyte
and the interferent. Two limiting
situations must be considered. First,
if the analyte
is the stronger acid or base,
then the titrant will begin reacting
with the analyte
before reacting with the interferent. The feasibility of the analysis depends
on whether the titrant’s reaction
with the in- terferent affects the accurate
location of the analyte’s equivalence point. If the acid
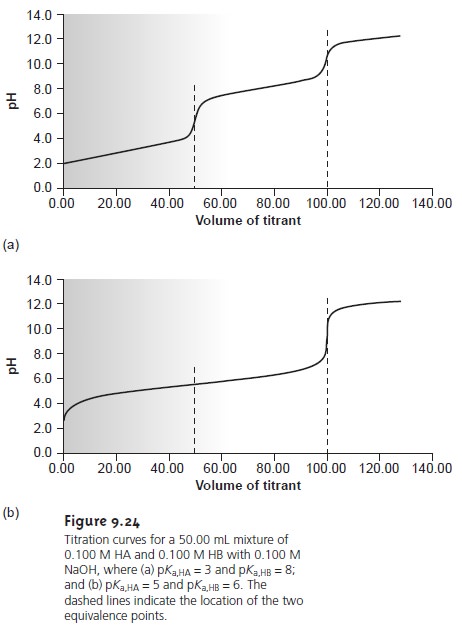
dissociation constants are substantially different, the end point for the analyte can be accurately determined (Figure
9.24a). Conversely, if the acid dissociation con- stants for the analyte
and interferent are similar, then an accurate
end point for the
analyte may not be found (Figure 9.24b).
In the latter case, a quantitative analysis for the analyte is not possible.
In the second
limiting situation the analyte is a weaker
acid or base than the in-
terferent. In this
case the volume
of titrant needed
to reach the
analyte’s equivalence point is determined by the concentration of both the analyte and the interferent. To account for the
contribution from the
interferent, an equivalence point for the
in- terferent must be present. Again,
if the acid dissociation constants for the analyte and interferent are significantly different, the analyte’s determination is possible. If, however, the acid dissociation constants are similar, only a single
equivalence point is found, and the analyte’s and interferent’s contributions to the equivalence point volume cannot be separated.
Time, Cost, and Equipment
Acid–base titrations require less time than most gravi- metric procedures, but more time than many instrumental methods of analysis, particularly when analyzing many samples.
With the availability of instruments for performing automated titrations, however, concerns about analysis time are less of
a problem. When performing a titration manually the equipment needs
are few (a buret and possibly a pH meter),
inexpensive, routinely available
in most laborato- ries, and easy
to maintain. Instrumentation for automatic titrations can be pur- chased for around $3000.
Related Topics