Chapter: Modern Analytical Chemistry: Chromatographic and Electrophoretic Methods
Supercritical Fluid Chromatography
Supercritical Fluid
Chromatography
Despite their importance, gas chromatography and liquid chromatography cannot be used to separate and analyze all types of samples. Gas chromatography, particu- larly when using capillary
columns, provides for rapid separations with excellent resolution. Its application, however,
is limited to volatile analytes
or those analytes that can be made volatile by a suitable
derivatization. Liquid chromatography can be used to separate a wider array
of solutes; however,
the most commonly
used de- tectors (UV, fluorescence, and electrochemical) do not respond
as universally as the
flame ionization detector commonly used in gas chromatography.
Supercritical fluid chromatography (SFC) provides
a useful alternative to gas
chromatography and liquid chromatography for some samples. The mobile phase in
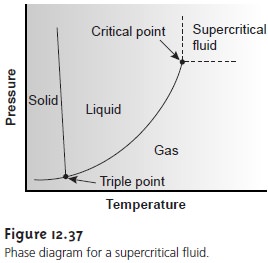
supercritical fluid chromatography is a gas held at a temperature and pressure ex- ceeding its critical point (Figure 12.37).
Under these conditions the mobile phase is
neither a gas nor a liquid. Instead,
the mobile phase
is a supercritical fluid whose properties are intermediate between
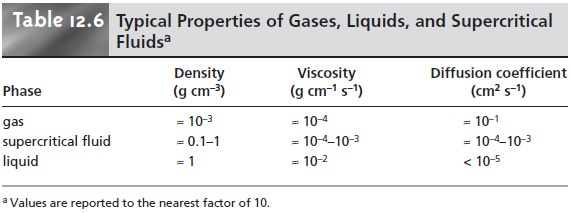
those of a gas and a liquid
(Table 12.6). Specifi- cally, supercritical fluids have viscosities that are similar
to those of gases, which means that they can move through
either capillary or packed columns
without the need for the high pressures encountered in HPLC. Analysis
time and resolution, al- though not as good as in GC,
are usually better
than that obtainable with conven-
tional HPLC. The density of a supercritical fluid, however, is much closer
to that of a liquid, accounting for its ability
to function as a solvent.
The mobile phase
in SFC, therefore, behaves
more like the liquid mobile phase in HPLC than the gaseous
mo- bile phase in GC.
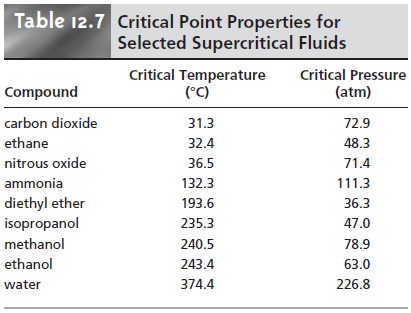
The most common mobile phase for supercritical fluid chromatography is CO2. Its low critical temperature, 31 °C, and critical pressure, 72.9 atm,
are rela- tively easy to achieve
and maintain. Although
supercritical CO2 is a good solvent for nonpolar organics, it is less useful for polar solutes. The addition of an organic modifier, such as methanol,
improves the mobile phase’s elution strength. Other common mobile
phases and their
critical temperatures and pressures are listed in Table
12.7.
The instrumentation necessary for supercritical fluid chromatography is essentially the
same as that
for a stan- dard GC or HPLC. The only important
addition is the need
for a pressure restrictor to maintain the critical pressure. Gradient elutions, similar to those in HPLC, are accom-
plished by changing the applied
pressure over time.
The re- sulting change in the density
of the mobile phase affects
its solvent strength.
Detection can be accomplished using stan-
dard GC detectors or HPLC detectors.
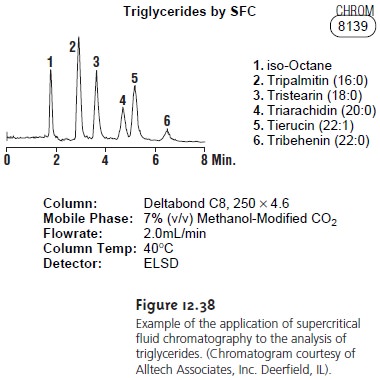
Supercritical fluid chromatography has found many applications in the analysis
of polymers, fossil
fuels, waxes, drugs, and
food products. Its
application in the
analysis of triglycerides is shown in Figure 12.38.
Related Topics