Chapter: Modern Analytical Chemistry: Chromatographic and Electrophoretic Methods
Gas Chromatography: Stationary Phases
Stationary Phases
Selectivity in gas chromatography is influenced by the choice
of stationary phase. Elution order in GLC
is determined primarily by the solute’s boiling point and,
to a lesser degree,
by the solute’s interaction with the stationary phase. Solutes with significantly different boiling points
are easily separated. On the other
hand, two solutes with similar boiling
points can be separated only if the stationary phase se-
lectively interacts with
one of the
solutes. In general, nonpolar solutes are
more easily separated with a nonpolar
stationary phase, and polar solutes
are easier to separate using a polar
stationary phase.
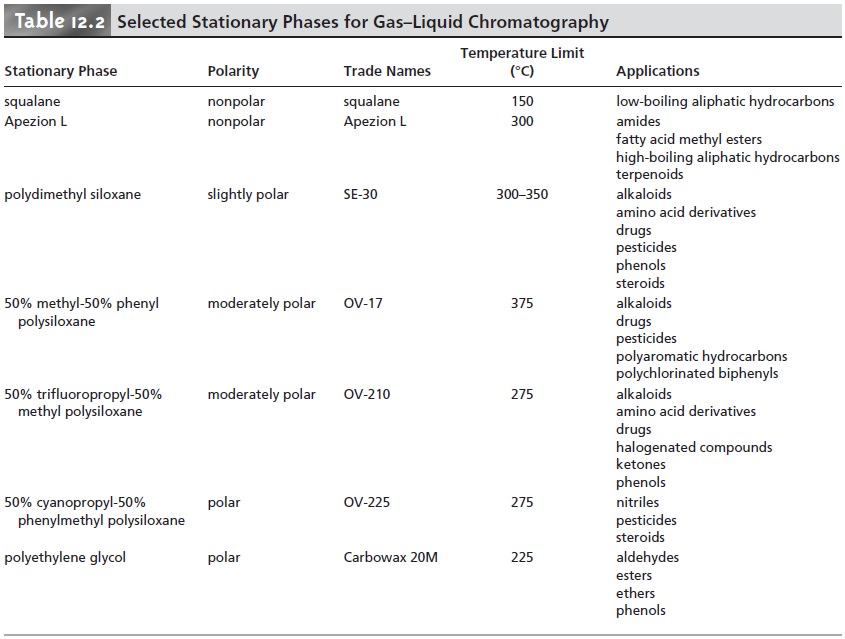
The main criteria for selecting a stationary phase are that it should be chemi- cally inert, thermally stable, of low volatility, and of an appropriate polarity for the solutes being separated. Although hundreds of stationary phases have been devel- oped, many of which are commercially available, the majority of GLC separations are accomplished with perhaps five to ten common stationary phases. Several of these are listed in Table 12.2, in order of increasing polarity, along with their physi- cal properties and typical applications.
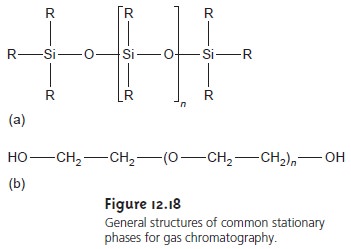
Many stationary phases have the general structure
shown in Figure 12.18a. A stationary phase of polydimethyl siloxane, in which
all the –R groups are methyl
groups (–CH3), is nonpolar and often makes
a good first choice for a new separa-
tion. The order of elution
when using polydimethyl siloxane usually follows
the boiling points of the solutes,
with lower boiling
solutes eluting first.
Replacing some of the
methyl groups with
other substituents increases the stationary phase’s
polar- ity, providing greater
selectivity. Thus, in 50% methyl-50% phenyl polysiloxane,
50% of the –R groups
are phenyl groups
(–C6H5), producing a slightly polar
sta- tionary phase. Increasing
polarity is provided by
substituting trifluoropropyl (–C3H6CF3) and cyanopropyl (–C3H6CN) functional groups or using
a stationary phase based
on polyethylene glycol
(Figure 12.18b).
An important problem
with all liquid
stationary phases is their tendency to “bleed” from the column.
The temperature limits
listed in Table
12.2 are those
that minimize the loss of stationary phase. When operated
above these limits,
a col- umn’s useful
lifetime is significantly shortened. Capillary columns
with bonded or cross-linked stationary phases provide
superior stability. Bonded stationary phases are attached to the capillary’s silica surface. Cross- linking, which is done
after the stationary phase is placed
in the capillary column,
links together separate polymer chains, thereby
providing greater stability.
Another important characteristic of a gas chromatographic column is the thickness of the stationary phase. As shown
in equa- tion 12.25,
separation efficiency improves with thinner films. The most common
film thickness is 0.25 ÎĽm. Thicker films
are used for highly volatile
solutes, such as gases, because
they have a greater
capacity for retaining such solutes. Thinner
films are used
when separating solutes of low volatility, such as steroids.
A few GLC stationary phases rely on chemical selectivity. The most notable
are stationary phases containing chiral functional groups,
which can be used for sepa-
rating enantiomers.6
Related Topics