Chapter: Modern Analytical Chemistry: Chromatographic and Electrophoretic Methods
Overview of Analytical Separations
Overview of Analytical Separations
We examined several
methods for separating an analyte from potential
interferents. For example,
in a liquid–liquid extraction the analyte and interferent
are initially present
in a single liquid phase.
A second, immiscible liquid phase is in-
troduced, and the two phases
are thoroughly mixed
by shaking. During
this process the analyte
and interferents partition themselves between the two phases
to differ- ent extents,
affecting their separation. Despite the power
of these separation tech- niques, there are some significant limitations.
The Problem with Simple Separations
Suppose we have a sample
containing an analyte
in a matrix that is incompatible
with our analytical method. To determine the analyte’s concentration we first sepa- rate it from the matrix using,
for example, a liquid–liquid extraction. If there are additional analytes, we may need to use additional extractions to isolate
them from the analyte’s matrix. For a complex mixture
of analytes this quickly becomes
a te- dious process.
Furthermore, the extent
to which we can effect
a separation depends
on the distribution ratio of each species in the sample.
To separate an analyte from its ma- trix,
its distribution ratio
must be significantly greater than that for all other com- ponents in the matrix.
When the analyte’s
distribution ratio is similar to that of an-
other species, then a separation becomes impossible. For example, let’s
assume that an analyte,
A, and a matrix interferent, I, have distribution ratios of 5 and 0.5, re-
spectively. In an attempt to separate the
analyte from its
matrix, a simple
liquid– liquid extraction is carried out using equal
volumes of sample
and a suitable extrac-
tion solvent. Following the treatment
outlined, it is easy to show that a
single extraction removes
approximately 83% of the analyte
and 33% of the inter- ferent. Although it is possible to remove 99%
of A with three extractions, 70% of I is also removed.
In fact, there is no practical combination of number of extractions
or volume ratio of sample
and extracting phases
that produce an acceptable separa- tion of the analyte
and interferent by a simple
liquid–liquid extraction.
A Better Way to Separate Mixtures
The problem with a simple
extraction is that the separation only occurs in one di- rection. In a liquid–liquid extraction, for example,
we extract a solute from its ini- tial phase into the extracting phase. Consider, again, the separation of an analyte and a matrix interferent with distribution ratios of 5 and 0.5, respectively. A single
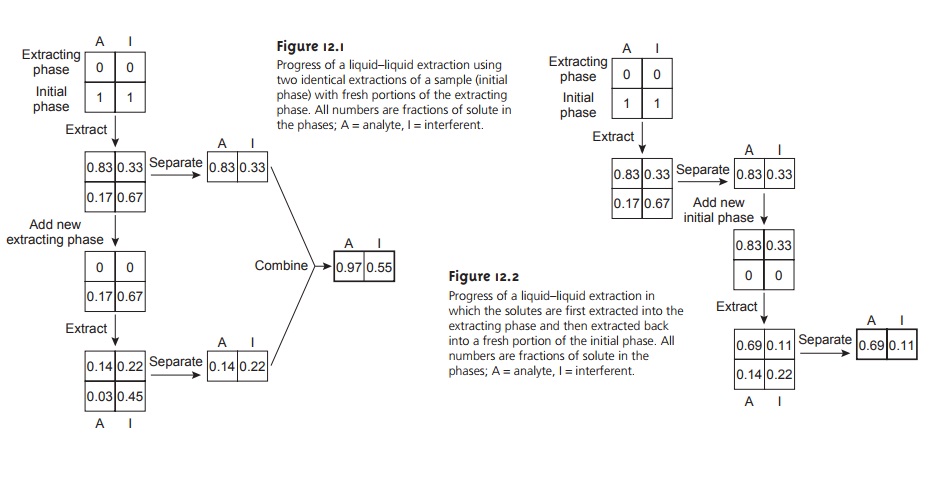
liquid–liquid extraction transfers 83% of the analyte and 33% of the interferent to the extracting phase
(Figure 12.1). If the concentrations of A and I in the sample were identical, then their concentration ratio in the extracting phase after one extraction is
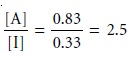
Thus, a single
extraction improves the
separation of the
solutes by a factor of 2.5. As shown in Figure 12.1,
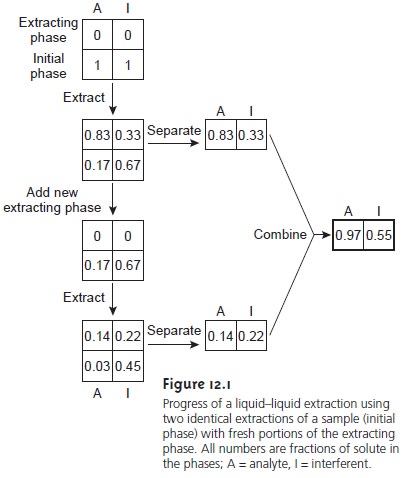
a second extraction actually leads to a poorer
separation. After combining the
two portions of the extracting phase, the concentration ratio decreases to
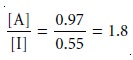
We can improve the separation by first extracting the solutes into the extracting phase, and then extracting them back into a fresh portion of the initial phase (Figure 12.2). Because solute A has the larger distribution ratio, it is extracted to a greater extent during the first extraction and to a lesser extent during the second ex- traction. In this case the final concentration ratio of in the extracting phase is significantly greater.
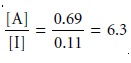
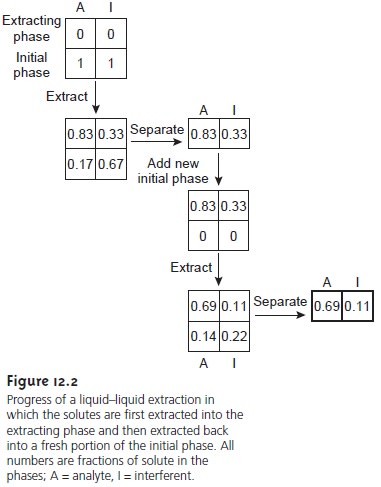
The process
of extracting the solutes
back and forth between fresh
portions of the
two phases, which
is called a counter- current extraction, was developed by Craig in the 1940s.1* The same phenomenon
forms the basis of modern
chromatography.
Chromatographic separations are accomplished by continuously
passing one sample-free phase, called a mobile phase,
over a second sample-free phase that re- mains
fixed, or stationary. The sample is injected, or placed, into the mobile phase.
As it moves with the mobile phase,
the sample’s components partition themselves
between the mobile and stationary phases.
Those components whose
distribution ratio favors the stationary phase
require a longer
time to pass through the system.
Given sufficient time,
and sufficient stationary and mobile phase,
solutes with simi- lar distribution ratios can
be separated.
The history of modern chromatography can be traced
to the turn of the cen-
tury when the Russian botanist
Mikhail Tswett (1872–1919) used a column
packed with a stationary phase of calcium
carbonate to separate colored pigments from plant extracts. The sample was placed at the top of the column and carried
through the stationary phase
using a mobile
phase of petroleum ether. As the sample moved through the column, the
pigments in the
plant extract separated into individual col- ored bands. Once the pigments were adequately separated, the calcium carbonate was removed from the column, sectioned, and the pigments
recovered by extrac- tion. Tswett named the technique chromatography, combining the Greek words for
“color” and “to write.” There
was little interest
in Tswett’s technique until 1931 when chromatography was reintroduced as an analytical technique for biochemical separations. Pioneering work by Martin
and Synge in 19412 established the impor- tance of liquid–liquid partition chromatography and led
to the development of a theory for
chromatographic separations; they
were awarded the
1952 Nobel Prize
in chemistry for this work. Since
then, chromatography in its many forms has become
the most important and widely used separation technique. Other separation meth- ods, such as electrophoresis, effect
a separation without
the use of a stationary phase.
Classifying Analytical Separations
Analytical separations may be classified in three ways:
by the physical state of the
mobile phase and stationary phase;
by the method of contact
between the mobile phase and stationary phase;
or by the chemical or physical mechanism responsible for separating the sample’s constituents. The mobile phase is usually
a liquid or a
gas, and the stationary phase,
when present, is a solid
or a liquid film coated
on a solid surface.
Chromatographic techniques are often named by listing the type of mobile phase,
followed by the
type of stationary phase. Thus, in gas–liquid chro- matography the mobile phase
is a gas and the
stationary phase is a liquid.
If only one phase
is indicated, as in gas chromatography, it is assumed
to be the mobile phase.
Two common approaches are used to bring the mobile phase and stationary phase into contact. In column chromatography, the stationary phase is placed in a narrow column through which the mobile phase moves under the influence of gravity or pressure. The stationary phase is either a solid or a thin, liquid film coating on a solid particulate packing material or the column’s walls. In planar chromatography the stationary phase coats a flat glass, metal, or plastic plate and is placed in a developing chamber. A reservoir containing the mobile phase is placed in contact with the stationary phase, and the mobile phase moves by capillary action.
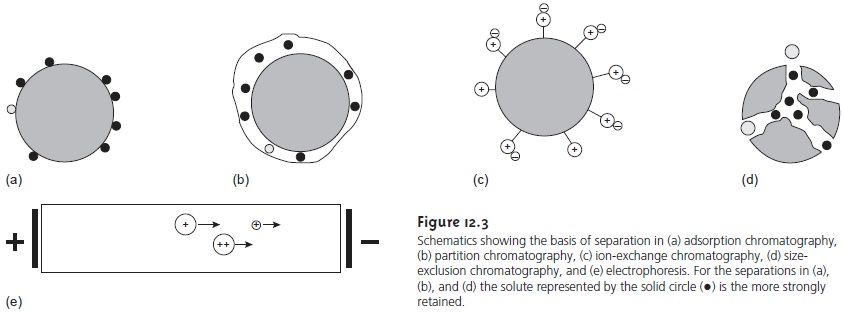
The mechanism by which solutes
separate provides a third means
for charac- terizing a
separation (Figure 12.3). In adsorption chromatography, solutes sepa- rate based
on their ability
to adsorb to a solid
stationary phase. In partition chro- matography, a thin liquid film coating
a solid support
serves as the stationary
phase. Separation is based on a difference
in the equilibrium partitioning of solutes between the liquid stationary phase and the mobile
phase. Stationary phases consisting of a solid
support with covalently attached anionic (e.g.,
–SO3–) or cationic (e.g., –N(CH3)3+) functional groups are used in ion-exchange chro- matography. Ionic solutes
are attracted to the stationary phase by electrostatic forces. Porous gels are
used as stationary phases in size-exclusion chromatogra- phy, in which separation is due to differences in the size of the solutes. Large solutes are
unable to penetrate into the porous
stationary phase and
so quickly pass through
the column. Smaller
solutes enter into the porous
stationary phase, increasing the time spent
on the column. Not all separation methods
require a stationary phase.
In an electrophoretic separation, for example, charged solutes migrate under
the influence of an applied
potential field. Differences in the mo- bility of the ions account for their separation.
Related Topics