Chapter: Modern Analytical Chemistry: Chromatographic and Electrophoretic Methods
Capacity Factor - Theory of Column Chromatography
Capacity Factor
The distribution of a solute,
S, between the mobile phase
and stationary phase
can be represented by an equilibrium reaction
Sm < = = = = > Ss
and its associated partition coefficient, KD, and distribution ratio, D,
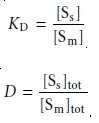 12.2
12.2
where the subscripts m and s refer to the mobile
phase and stationary phase, respec-
tively. As long as the
solute is not
involved in any
additional equilibria in either the mobile phase or stationary phase, the equilibrium partition coefficient and the dis- tribution ratio will be the same.
Conservation of mass requires that the total moles of solute
remain constant throughout the separation, thus
(moles S)tot = (moles S)m + (moles S)s …………..12.3
Solving equation 12.3 for the moles of solute in the stationary phase and substitut- ing into equation 12.2 gives
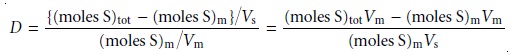
where Vm and Vs are the volumes
of the mobile and stationary phases. Rearranging
and solving for the fraction of solute in the mobile
phase, fm, gives
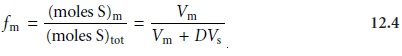
Note that this equation is identical to that describing the extraction of a solute
in a liquid–liquid extraction. Since the volumes
of the sta- tionary and mobile
phase may not be known,
equation 12.4 is simplified by dividing
both the numerator and denominator by Vm; thus
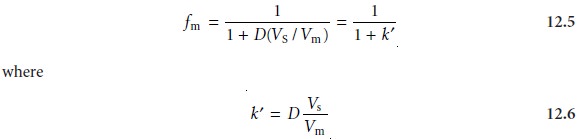
is the solute’s capacity factor.
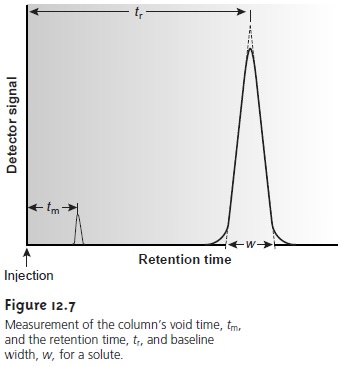
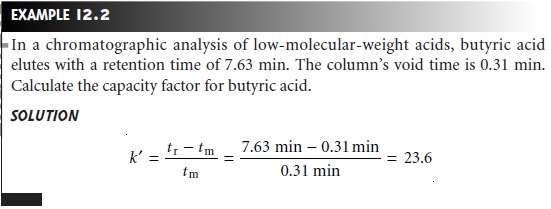
A solute’s capacity
factor can be determined from a chromatogram by measur- ing the column’s void time, tm, and the solute’s
retention time, tr (see
Figure 12.7). The mobile
phase’s average linear
velocity, u, is equal
to the length of the column, L, divided by the time
required to elute
a nonretained solute.
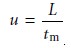 12.7
12.7
By the same reasoning, the solute’s average
linear velocity, v, is
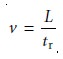 12.8
12.8
The solute can
only move through
the column when
it is in the mobile
phase. Its average linear velocity, therefore, is simply the product of the mobile phase’s aver- age
linear velocity and the fraction
of solute present
in the mobile phase.
v =
ufm ………………12.9
Substituting equations 12.5, 12.7, and 12.8 into equation 12.9
gives
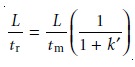
Finally, solving this equation for k’ gives
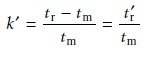 12.10
12.10
where tr’ is known as the adjusted retention time.
Related Topics