Chapter: Modern Analytical Chemistry: Chromatographic and Electrophoretic Methods
Gas Chromatography: Chromatographic Columns
Chromatographic Columns
A chromatographic column provides a location for physically retaining
the station- ary phase.
The column’s construction also influences the
amount of sample
that can be handled, the efficiency of the separation, the number of analytes that
can be eas- ily separated, and the amount of time required
for the separation. Both packed and capillary columns are used
in gas chromatography.
Packed Columns
A packed column is constructed from glass, stainless steel,
copper or aluminum
and is typically 2–6 m in length,
with an internal
diameter of 2–4 mm. The column
is filled with a particulate solid support, with particle diam- eters ranging from 37–44 μm to 250–354 μm.
The most widely
used particulate support
is diatomaceous earth,
which is com- posed of the silica skeletons of diatoms. These particles are quite porous,
with sur- face areas
of 0.5–7.5 m2/g,
which provides ample
contact between the mobile phase and stationary phase. When hydrolyzed, the surface of a diatomaceous earth con- tains silanol
groups (–SiOH), providing
active sites that absorb solute molecules in gas–solid chromatography.
In gas–liquid
chromatography (GLC), separation is based on the partitioning of solutes between a gaseous mobile
phase and a liquid stationary phase coated on the
solid packing material.
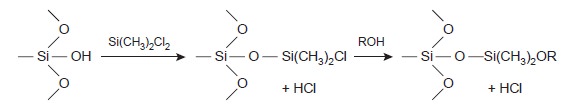
To avoid the adsorption of solute molecules
on exposed packing material, which degrades the quality of the separation, surface silanols are deactivated by silanizing with dimethyldichlorosilane and washing with an alcohol (typically methanol) before coating
with stationary phase.
More recently, solid
supports made from
glass beads or fluorocarbon polymers have been introduced. These
supports have the
advantage of being
more inert than
di- atomaceous earth.
To minimize the multiple path and mass transfer contributions to plate height (equations 12.23 and 12.26),
the packing material
should be of as small a diameter as is practical and loaded with a thin film of stationary phase
(equation 12.25). Compared with capillary columns,
which are discussed
in the next section, packed columns can handle larger
amounts of sample.
Samples of 0.1–10
ÎĽL are routinely analyzed with a packed column.
Column efficiencies are typically several
hundred to 2000 plates/m, providing columns with
3000–10,000 theoretical plates.
Assuming Vmax/Vmin is approximately 50, a packed
column with 10,000
theoretical plates has a
peak capacity (equation 12.18) of
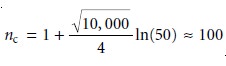
Capillary Columns
Capillary, or open tubular columns are constructed from fused silica coated
with a protective polymer. Columns
may be up to 100 m in length with an internal
diameter of approximately 150–300 μm (Figure
12.17). Larger bore columns
of 530 ÎĽm, called megabore
columns, also are available.

Capillary columns are of
two principal
types. Wall-coated open tubular columns (WCOT) contain a thin layer of stationary phase,
typically 0.25 ÎĽm thick,
coated on the capillary’s inner wall. In support-coated open tubular columns
(SCOT), a thin layer of a solid
support, such as a diatomaceous earth, coated with a
liquid stationary phase
is attached to the capillary’s inner wall.
Capillary columns provide
a significant improvement in separation efficiency. The pressure needed to move the mobile phase through a packed column limits its length. The absence of packing material
allows a capillary
column to be longer than a
packed column. Although
most capillary columns
contain more theoretical plates per meter than a packed
column, the more important contribution to their greater efficiency is the ability
to fashion longer
columns. For example, a 50-m capillary column with 3000 plates/m
has 150,000 theoretical plates and, assuming
Vmax/Vmin is approximately 50,3 a peak
capacity of almost
380. On the
other hand, packed columns can handle larger samples. Due to its smaller diameter,
capillary columns require smaller
samples; typically less
than 10–2 μL.
Related Topics