Chapter: Modern Analytical Chemistry: Chromatographic and Electrophoretic Methods
Ion-Exchange Chromatography
Ion-Exchange
Chromatography
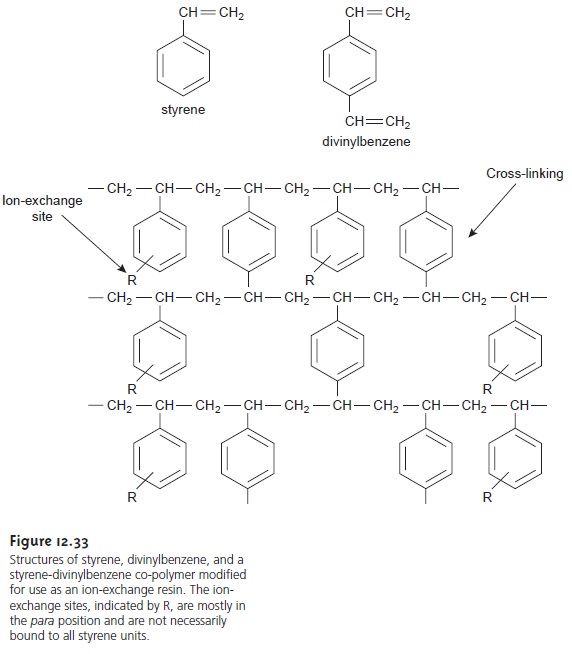
In ion-exchange chromatography (IEC) the stationary phase
is a cross-linked poly-
mer resin, usually divinylbenzene cross-linked polystyrene, with covalently attached ionic functional groups
(Figure 12.33). The counterions to these fixed
charges are mobile and can be displaced by ions that compete more favorably for the exchange sites. Ion-exchange resins are divided into four categories: strong acid cation
ex- changers; weak acid cation exchangers; strong base anion exchangers;
and weak base anion exchangers. Table
12.5 provides a list of several common
ion-exchange resins.
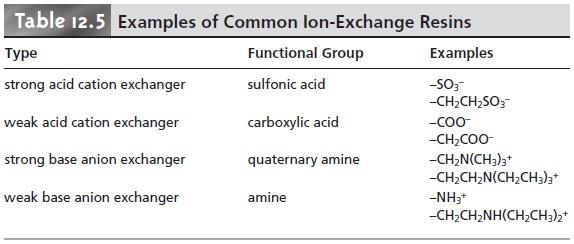
Strong acid cation exchangers include a sulfonic acid functional group that re- tains its anionic form, and thus its capacity for ion-exchange, in strongly acidic so- lutions. The functional groups for a weak acid cation exchanger, however, are fully protonated at pH levels less then 4, thereby losing their exchange capacity. The strong base anion exchangers are fashioned using a quaternary amine, therefore re- taining a positive charge even in strongly basic solutions. Weak base anion exchang- ers, however, remain protonated only at pH levels that are moderately basic. Under more basic conditions, a weak base anion exchanger loses its positive charge and, therefore, its exchange capacity.
The ion-exchange reaction
of a monovalent cation, M+, at a strong acid ex-
change site is
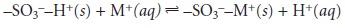
The equilibrium constant
for this ion-exchange reaction, which is also called
the se- lectivity
coefficient, is
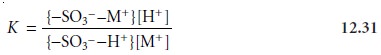
where the brackets
{ } indicate a surface
concentration. Rearranging equation
12.31 shows that the distribution ratio
for the exchange
reaction
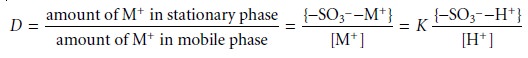
is a function
of the concentration of H+ and, therefore, the pH of the mobile
phase.
Ion-exchange resins are incorporated into HPLC columns
either as micron- sized porous
polymer beads or by coating
the resin on porous silica
particles. Selec- tivity is somewhat dependent on whether the resin includes
a strong or weak ex- change site and on the extent
of cross-linking. The latter is particularly important because it controls the resin’s permeability and, therefore, the accessibility of the ex- change sites. An approximate order of selectivity for a typical
strong acid cation
ex- change resin, in order of decreasing D, is
Al3+ > Ba2+ > Pb2+ > Ca2+ > Ni2+ > Cd2+ > Cu2+ > Co2+ > Zn2+ > Mg2+
|
4 |
Note that highly charged ions bind more strongly than ions of lower charge. Within a group of ions of similar charge, those ions with a smaller hydrated radius or those that are more polarizable bind more strongly. For a strong base anion exchanger the general order is
SO42– > I– > HSO4– > NO3– > Br– > NO3– > Cl– > HCO3– > CH3COO– > OH– > F–
Again, ions of higher charge and smaller
hydrated radius bind more strongly
than ions with a lower charge
and a larger hydrated radius.
The mobile phase
in IEC is usually an aqueous buffer,
the pH and ionic com- position of which determines a solute’s retention time. Gradient elutions
are possi- ble in which the
ionic strength or pH of the mobile
phase is changed
with time. For example, an IEC separation of cations might
use a dilute solution of HCl as the mo- bile
phase. Increasing the concentration of HCl speeds
the elution rate for more strongly retained cations, since
the higher concentration of H+ allows it to compete more successfully for the
ion-exchange sites.
Ion-exchange columns can be substituted into the general HPLC
instrument shown in Figure
12.26. The most common detector
measures the conductivity of the mobile phase
as it elutes from the column. The high concentration of electrolyte in the mobile phase
is a problem, however, because
the mobile-phase ions dominate
the conductivity. For example, if a dilute solution of HCl is used as the mobile phase, the presence of large concentrations of H3O+ and Cl– produces a background
conductivity that may prevent the detection of analytes eluting
from the column.
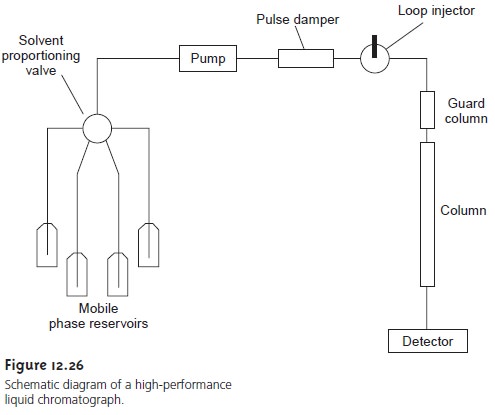
To minimize the mobile phase’s
contribution to conductivity, an ion-suppressor column is placed between
the analytical column
and the detector. This column se- lectively removes mobile-phase
electrolyte ions without removing solute ions. For example, in cation ion-exchange chromatography using a dilute solution
of HCl as the mobile phase,
the suppressor column
contains an anion-exchange resin. The ex- change reaction
H+(aq) + Cl–(aq) + Resin+–OH– < =
= = = > Resin+–Cl– + H2O(l)
replaces the ionic HCl with H2O. Analyte
cations elute as hydroxide salts instead of as chloride salts. A similar
process is used in anion ion-exchange chromatography in which a cation ion-exchange resin is placed in the suppressor column.
If the mo- bile phase contains Na2CO3,
the exchange reaction
2Na+(aq) + CO32–(aq)
+ 2Resin––H+ < = = = = > 2Resin––Na+ +H3CO
(aq)
replaces a strong electrolyte, Na2CO3,
with a weak electrolyte, H2CO3.
Ion suppression is necessary when using a mobile phase
containing a high con-
centration of ions. Single-column ion
chromatography, in which an ion-suppressor column is not needed,
is possible if the concentration of ions in the mobile
phase can be minimized. Typically this is done by using a stationary phase
resin with a low
capacity for ion
exchange and a mobile phase
with a small
concentration of ions. Because
the background conductivity due to the mobile phase is sufficiently small, it is possible to monitor a change in conductivity as the analytes elute from the column.
A UV/Vis absorbance detector can also
be used if the solute
ions absorb ultravi- olet or visible radiation. Alternatively, solutions that do not absorb in the UV/Vis range can be detected indirectly
if the mobile phase contains a UV/Vis-absorbing
species. In this case, when a solute
band passes through
the detector, a decrease in absorbance is measured at the detector.
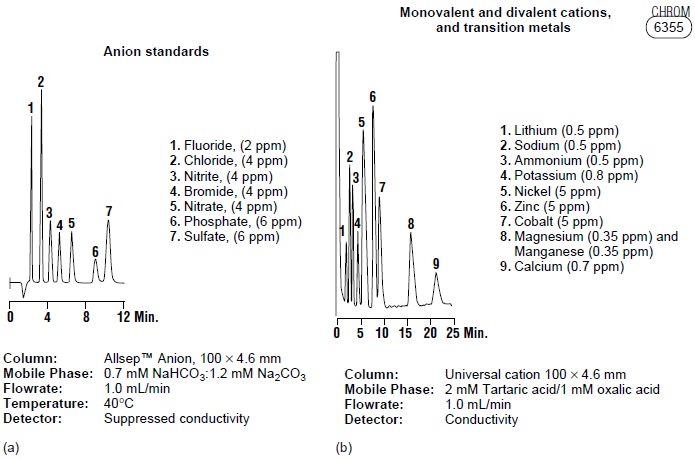
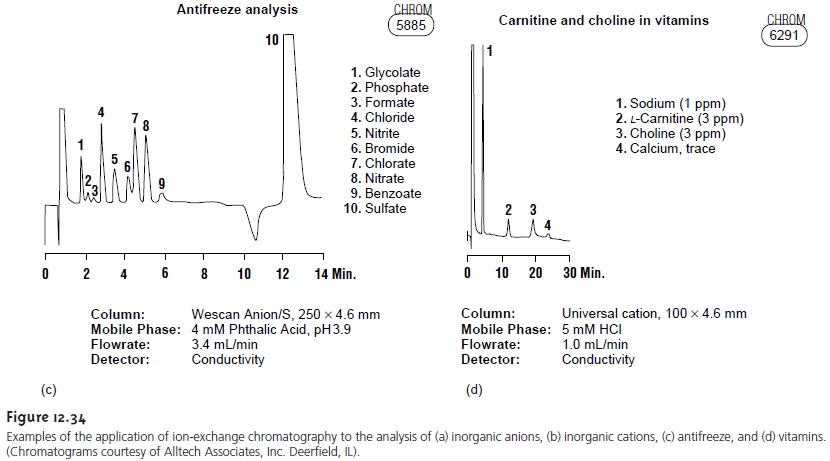
Ion-exchange chromatography has found important applications in water analysis and in biochemistry. For example, Figure
12.34a shows how ion-exchange
chromatography can be used for the simultaneous analysis of seven common an- ions in approximately 12 min. Before
IEC, a complete analysis of the same set of anions required 1–2 days.
Ion-exchange chromatography also has been used for the analysis of proteins, amino
acids, sugars, nucleotides, pharmaceuticals,
con- sumer products, and
clinical samples. Several
examples are shown
in Figure 12.34.
Related Topics