Chapter: Modern Analytical Chemistry: Chromatographic and Electrophoretic Methods
Optimizing Chromatographic Separations Using Column Efficiency to Optimize Resolution
Using Column Efficiency to Optimize Resolution
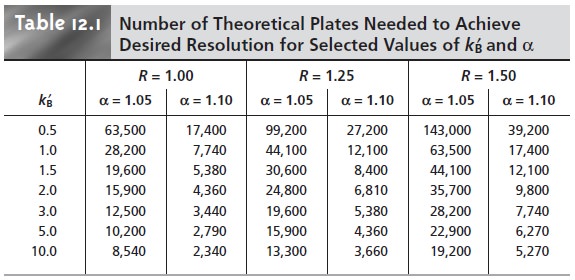
If the capacity factor and α are known, then equation 12.21 can be used to calculate the number of theoretical plates needed to achieve a desired resolution (Table 12.1). For example, given α = 1.05 and kB’ = 2.0, a resolution of 1.25 requires approxi- mately 24,800 theoretical plates. If the column only provides 12,400 plates, half of what is needed, then the separation is not possible. How can the number of theoret- ical plates be doubled? The easiest way is to double the length of the column; how- ever, this also requires a doubling of the analysis time. A more desirable approach is to cut the height of a theoretical plate in half, providing the desired resolution with- out changing the analysis time. Even better, if H can be decreased by more than 50%, it also may be possible to achieve the desired resolution with an even shorter analysis time by decreasing kB’ or α.
To determine how the height
of a theoretical plate can be decreased, it is neces- sary to understand the
experimental factors contributing to the broadening of a solute’s chromatographic band. Several theoretical treatments of band broadening
have been proposed. We will consider one approach in which the height of a theo- retical plate is determined by four contributions: multiple paths, longitudinal diffu- sion, mass transfer
in the stationary phase, and mass transfer
in the mobile phase.
Multiple Paths
Solute
molecules passing through a chromatographic column
travel separate paths
that may differ
in length. Because
of these differences in path length, solute
molecules injected simultaneously elute at different times. The princi- pal factor contributing to this variation in path length
is a nonhomogeneous pack-
ing of the stationary phase
in the column. Differences in particle size and packing consistency cause solute molecules
to travel paths of different
length. Some solute molecules follow relatively straight
paths through the column, but others follow
a longer, more tortuous path (Figure 12.14a). The contribution of
multiple paths to the height
of a theoretical plate, Hp, is
Hp = 2λdp………………12.23
where dp is
the average diameter of the particulate packing material, and λ is a con-stant accounting for the consistency of the packing.
A smaller range
of particle sizes and a more consistent packing produce a smaller value
for λ. Note that for an open tubular column, which does
not contain packing
material, Hp is 0.
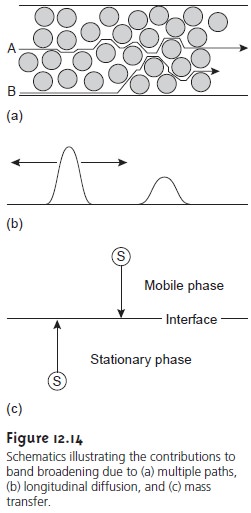
Longitudinal Diffusion
The
second contribution to band broadening is the result of the solute’s longitudinal diffusion in the mobile
phase. Even if the mobile
phase velocity is 0, solute molecules
are constantly in motion, diffusing
through the mo- bile
phase. Since the concentration of solute is greatest at the center of a chromato-
graphic band, more solute diffuses
toward the band’s
forward and rear edges than diffuses toward the band’s center. The net result is an increase in the band’s width
(Figure 12.14b). The contribution of longitudinal diffusion to the height
of a theo- retical plate, Hd, is

where Dm is the solute’s diffusion coefficient in the mobile
phase, u is the
mobile phase velocity, and Îł is a constant related to the
column packing. The
effect of Hd
on the height of a theoretical plate
is minimized by a high
mobile-phase velocity.
Because a solute’s diffusion coefficient is larger in a gaseous
mobile phase than in
a liquid mobile phase, longitudinal diffusion is a more serious
problem in gas chromatography.
Mass Transfer
The final two contributions to band broadening result from the fi-
nite time required for a solute molecule
to diffuse through
the stationary phase and
mobile phase. A chromatographic separation occurs because solutes
move between the stationary and mobile phases.
For a solute to move from one phase to the other, it
must first diffuse
to the interface between the two phases
(Figure 12.14c)—a process
called mass transfer. A
contribution to band broadening occurs
whenever the solute’s movement
to the interface is not fast enough
to maintain a true equilib- rium distribution of solute
between the two phases. Thus,
solute molecules in the
mobile phase move farther down the column
than expected before
passing into the stationary phase. Solute molecules in the stationary phase, on the other hand,
take longer than expected
to cross into the mobile
phase. The contributions of mass transfer in the stationary phase, Hs, and mass transfer
in the mobile phase, Hm, are given by
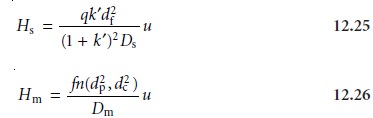
where df is the thickness of the stationary phase, dc is the column’s
diameter, Ds is the solute’s diffusion coefficient in the
stationary phase, q is
a constant related
to the column packing
material, and the remaining terms
are as previously defined. As in-
dicated in equation 12.26, the
exact form of Hm is unknown, although it is a func-
tion of particle size and column diameter.
The contribution of mass transfer
to the height of a theoretical plate is smallest
for slow mobile-phase velocities, smaller di- ameter packing materials, and
thinner films of stationary phase.
Putting It All Together
The net height
of a theoretical plate is a summation of the
contributions from each of the terms in equations 12.23–12.26; thus,
H =
Hp+
Hd + Hs + Hm………………12.27
An alternative form of this equation is the van Deemter equation

which emphasizes the importance of the mobile phase’s flow rate. In the van Deemter equation, A accounts for multiple paths (Hp), B/u for longitudinal diffusion (Hd), and Cu for the solute’s mass transfer in the stationary and mobile phases (Hs and Hm).
There is some disagreement on the correct
equation for describing the relation- ship between
plate height and mobile-phase velocity.
In addition to the van Deemter equation
(equation 12.28), another
equation is that proposed by Hawkes
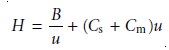
where Cs and Cm are the mass transfer terms
for the stationary and mobile phases respectively. A third equation
was devised by Knox.
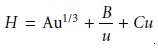
All three equations, and others, have been used to characterize
chromatographic systems, with no single equation
providing the best explanation in every case.
To increase the number of theoretical plates
without increasing the length of the
column, it is necessary to decrease one or more of the terms in equation 12.27 or
equation 12.28. The easiest way to accomplish this is by adjusting the velocity of the
mobile phase. At a low mobile-phase velocity, column efficiency is limited by longitudinal diffusion, whereas at
higher velocities efficiency is limited by the
two mass transfer terms.
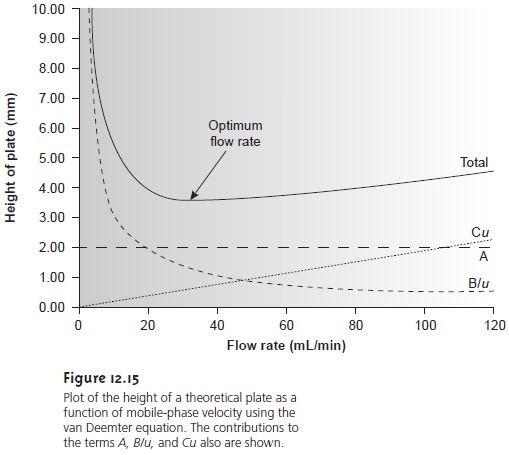
As shown in Figure 12.15
(which is interpreted in terms of equation 12.28), the optimum
mobile-phase velocity corresponds to a minimum
in a plot of H as a function of u.
The remaining parameters affecting the height
of a theoretical plate are deter-
mined by the construction of the column
and suggest how the column’s
design may be used
to improve efficiency. For example, both
Hp and
Hm are
a function of the
size of the particles used for the packing material.
Decreasing particle size, therefore, is one approach
to improving efficiency. A decrease in particle size is
limited, however, by the need for a greater pressure to push the mobile phase through
the column.
One of the most important advances in column construction has been the de- velopment of open tubular, or capillary columns that contain no packing material (dp = 0). Instead, the interior wall of a capillary column is coated with a thin film of the stationary phase. The absence of packing material means that the mobile phase can move through the column with substantially less pressure.
As a result,
capillary columns can be manufactured with
much greater lengths
than is possible with a packed column.
Furthermore, plate height is reduced
because the Hp term in equation 12.27 disappears and the Hm term
becomes smaller. The combination of a
smaller height for a theoretical plate and a longer column leads to an approximate 100-fold increase
in the number of theoretical plates. Capillary columns
are not without disadvantages. Because capillary columns
are much narrower than packed
columns, they require a significantly smaller amount of sample. Difficulties with re- producibly injecting small samples complicates the use of capillary chromatography for quantitative work.
Another approach to improving resolution is to use thin films
of stationary phase. Capillary columns used in gas chromatography and the bonded
phases com- monly used in HPLC provide a significant decrease
in plate height due to the reduc- tion of the Hs term in equation
12.27.
Related Topics