Chapter: Modern Analytical Chemistry: Chromatographic and Electrophoretic Methods
LiquidŌĆōSolid Adsorption Chromatography
LiquidŌĆōSolid
Adsorption Chromatography
In liquidŌĆōsolid adsorption chromatography (LSC) the column packing
also serves as the
stationary phase. In TswettŌĆÖs original work the stationary phase was finely
di- vided CaCO3, but modern columns
employ porous 3ŌĆō10-╬╝m
particles of silica
or alumina. Since the stationary phase
is polar, the mobile phase
is usually a nonpolar
or moderately polar solvent. Typical
mobile phases include
hexane, isooctane, and methylene chloride. The usual
order of elution, from shorter to longer retention times, is
olefins < aromatic hydrocarbons < ethers < esters,
aldehydes, ketones < alcohols, amines
< amides < carboxylic acids
For most samples liquidŌĆōsolid chromatography does not offer any
special advan- tages over liquidŌĆōliquid chromatography (LLC). One exception is for the analysis of isomers, where LLC excels.
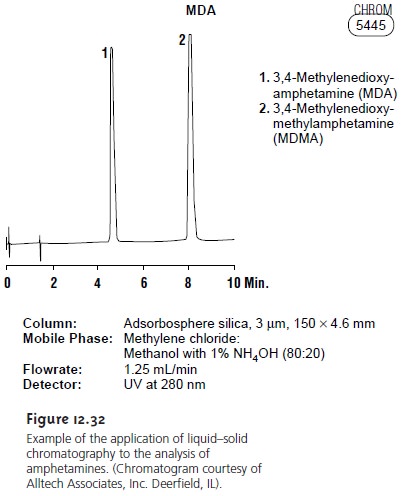
Figure 12.32 shows a typical
LSC separation of two am- phetamines on a silica column using an 80:20 mixture of methylene chloride
and methanol containing 1% NH4OH as a mobile phase. Nonpolar
stationary phases, such as charcoal-based absorbents, also may
be used.
Related Topics