Chapter: 11th Chemistry : UNIT 9 : Solutions
Osmosis and osmotic pressure
Osmosis and osmotic pressure
Many biological processes depend on osmosis, which is a spontaneous process by which the solvent molecules pass through a semi permeable membrane from a solution of lower concentration to a solution of higher concentration. The name osmosis is derived from the Greek word ŌĆśosmosŌĆÖ which means ŌĆśto pushŌĆÖ. It is also important to know that the semipermeable membrane selectively allows certain molecules in the solution to pass through it but not others.
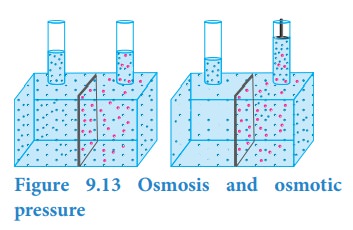
Let us consider a simple apparatus as shown in the above figure. A semipermeable membrane separates a chamber into two compartments. Water (pure solvent) is added to the first compartment and the aqueous NaCl (solution) is added to the second compartment such that the liquid levels on the both sides are equal. Since there is a difference in concentration between the liquids present in the two compartments, the water molecules move from first compartment to second compartment through the semipermeable membrane. The membrane allows only the water molecules to pass through it in either direction but not allows the NaCl. The net flow of water is into the sodium chloride solution and hence increases its volume. This decreases its concentration and also creates a pressure difference between the compartments. This pressure difference, push some of the water molecules back to the solvent side through the semipermeable membrane until an equilibrium is established. At the equilibrium, the rate of movement of solvent molecules on both directions are equal. The pressure difference at the equilibrium is called osmotic pressure (ŽĆ). Thus, osmotic pressure can be defined as ŌĆ£the pressure that must be applied to the solution to stop the influx of the solvent (to stop osmosis) through the semipermeable membraneŌĆØ
vanŌĆÖt Hoff found out that for dilute solutions, the osmotic pressure is directly proportional to the molar concentration of the solute and the temperature of the solution. He proposed the following equation to calculate osmotic pressure which is now called as vanŌĆÖt Hoff equation.
ŽĆ=cRT ...........9.31
Here,
c = Concentration of the solution in molarity
T = Temperature
R = Gas constant
Related Topics