Chapter: Modern Analytical Chemistry: Chromatographic and Electrophoretic Methods
High-Performance Liquid Chromatography (HPLC): Mobile Phases
Mobile Phases
The elution order
of solutes in HPLC is governed by polarity. In a normal-phase separation the least polar
solute spends proportionally less time in the polar
station- ary phase and is the first solute
to elute from the column.
Retention times are con-
trolled by selecting the mobile
phase, with a less polar
mobile phase leading
to longer retention times. If, for example, a separation is poor because
the solutes are eluting too quickly, switching to a less polar mobile
phase leads to longer retention times and more opportunity for an acceptable separation. When two solutes are ad-
equately resolved, switching to a more polar mobile
phase may provide
an accept- able separation with a shorter
analysis time. In a reverse-phase separation the order of elution is reversed, with the most polar solute
being the first
to elute. Increasing the polarity of the mobile phase leads to longer retention
times, whereas shorter
retention times require
a mobile phase
of lower polarity.
Choosing a Mobile Phase
Several indices have been developed to assist in selecting
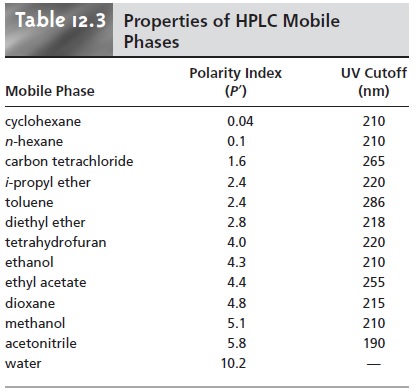
a mobile phase, the most useful of which is the polarity index.12 Table 12.3 provides
values for the polarity index, PŌĆÖ, of several
commonly used mobile phases, in which
larger values of PŌĆÖ correspond to more polar
solvents. Mobile phases
of intermedi- ate polarity
can be fashioned by mixing
together two or more of the mobile
phases in Table 12.3.
For example, a binary mobile
phase made by combining solvents A and B has a polarity
index, PAŌĆÖB, of

where PAŌĆÖ and PBŌĆÖ are the polarity indexes for solvents A and B, and ’ü”A and ’ü”B are the
volume fractions of the two solvents.
A useful guide
when using the polarity index
is that a change in its value
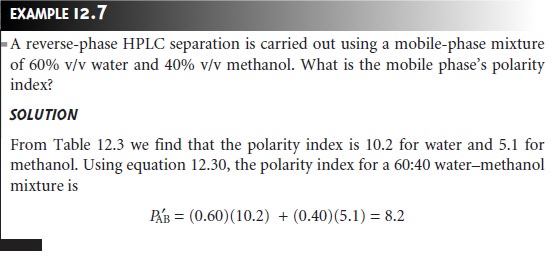
of 2 units corresponds to an approximate tenfold change in a soluteŌĆÖs
capacity factor. Thus, if kŌĆÖ is 22 for the reverse-phase separation of a solute
when using a mobile
phase of water (PŌĆÖ = 10.2), then switching to a 60:40
waterŌĆōmethanol mobile phase (PŌĆÖ = 8.2) will decrease kŌĆÖ to approximately 2.2. Note that the capacity
factor de- creases because
we are switching from a more polar
to a less polar mobile
phase in a reverse-phase separation.
Changing the mobile phaseŌĆÖs polarity index, by changing the relative amounts of two solvents, provides a means of changing a soluteŌĆÖs capacity factor. Such changes, however, are not very selective; thus, two solutes that significantly overlap may continue to be poorly resolved even after making a significant change in the mobile phaseŌĆÖs polarity.
To effect a better separation between two solutes
it is often necessary to im-
prove the selectivity factor, ╬▒. Two approaches are commonly used
to accomplish this improvement. When a solute
is a weak acid or a weak base, adjusting the pH of the
aqueous mobile phase
can lead to significant changes
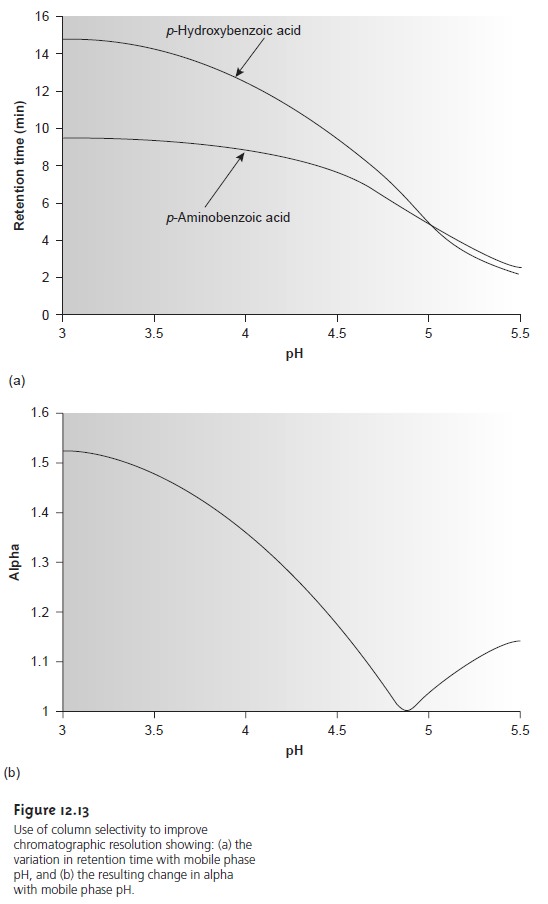
in the soluteŌĆÖs retention time. This is shown in Figure 12.13a for the reverse-phase separation of p- aminobenzoic acid and p-hydroxybenzoic acid on a nonpolar C18 column. At more acidic pH levels, both weak acids
are present as neutral molecules. Because they par- tition favorably into the
stationary phase, the
retention times for
the solutes are fairly long. When the pH is made more basic, the solutes, which
are now present
as their conjugate weak base anions,
are less soluble
in the stationary phase and elute
more quickly. Similar effects can be achieved
by taking advantage
of metalŌĆōligand complexation and
other equilibrium reactions.
A second approach
to changing the selectivity factor
for a pair of solutes
is to change one
or more of the mobile-phase solvents. In a reverse-phase separation, for example, this is accomplished by changing the solvent mixed with water. Besides
methanol, other common
solvents for adjusting retention times are acetonitrile and tetrahydrofuran (THF).
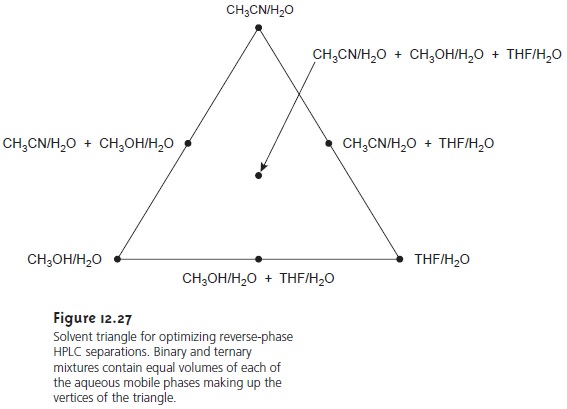
A common strategy
for finding the best mobile
phase is to use
the solvent triangle shown in Figure
12.27. The separation is first optimized using an aqueous mobile
phase of acetonitrile to produce the
best separation within the desired analysis time (methanol or THF also could be chosen first).
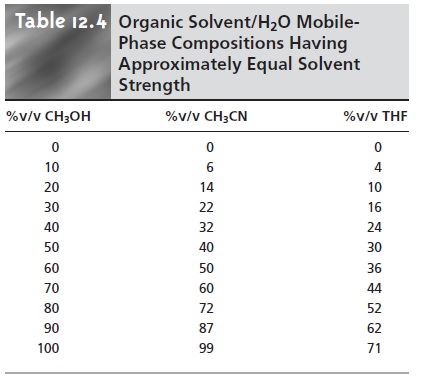
Table 12.4 is used to estimate the composition of
methanol/H2O and THF/H2O mobile phases that will produce similar
analysis times. These
mobile phases are then adjusted, if necessary, establishing the three points
of the solvent triangle. Four additional mo- bile
phases are prepared
using the binary
and ternary mobile
phases indicated in Figure 12.27. From these
seven mobile phases
it is possible to estimate how a change in the mobile-phase composition might affect the separation
Isocratic Versus Gradient Elution
When a separation uses a single mobile phase of fixed composition it is called an isocratic elution. It is often difficult, however, to find a single mobile-phase composition that is suitable for all solutes. Recalling the general elution problem, a mobile phase that is suitable for early eluting solutes may lead to unacceptably long retention times for later eluting solutes.
Optimizing conditions for late eluting solutes,
on the other hand, may provide an inadequate sepa- ration of early eluting
solutes. Changing the composition of the mobile phase with time
provides a solution
to this problem.
For a reverse-phase separation the initial
mobile-phase composition is relatively polar.
As the separation progresses, the mo-
bile phaseŌĆÖs composition is made less polar. Such separations are called gradient elutions.
Related Topics