Chemistry - Factors influencing the solubility of the solutes | 11th Chemistry : UNIT 9 : Solutions
Chapter: 11th Chemistry : UNIT 9 : Solutions
Factors influencing the solubility of the solutes
Factors influencing the solubility
The solubility of a solute generally depends on the nature of the solute and the solvent in which it is dissolved. It also depends on the temperature and pressure of the solution.
Nature of solute and solvent:
Sodium chloride, an ionic compound, dissolves readily in a polar solvent such as water, but it does not dissolve in non-polar organic solvents such as benzene or toluene. Many organic compounds dissolve readily in organic solvents and do not dissolve in water. Different gases dissolve in water to different extents: for example, ammonia is more soluble than oxygen in water.
Effect of temperature:
Solid solute in liquid solvent:
Generally, the solubility of a solid solute in a liquid solvent increases with increase in temperature. When the temperature is increased, the average kinetic energy of the molecules of the solute and the solvent increases. The increase in kinetic energy facilitates the solvent molecules to break the intermolecular attractive forces that keep the solute molecules together and hence the solubility increases.
When a solid is added to a solvent, it begins to dissolve. i.e. the solute leaves from the solid state (dissolution). After some time, some of the dissolved solute returns back to the solid state (recrystallisation). If there is excess of solid present, the rate of both these processes becomes equal at a particular stage. At this stage an equilibrium is established between the solid solute molecules and dissolved solute molecules.
Solute (solid) ⇆ Solute (dissolved)
According to Le-Chatelier principle, if the dissolution process is endothermic, the increase in temperature will shift the equilibrium towards left i.e solubility increases. for an exothermic reaction, the increase in temperature decreases the solubility. The solubilities of ammonium nitrate, calcium chloride, ceric sulphate nano-hydrate and sodium chloride in water at different temperatures are given in the following graph.
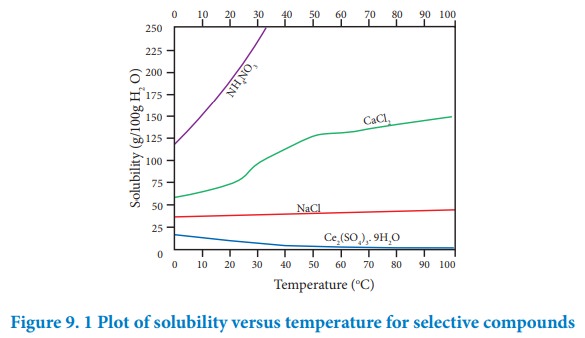
The following conclusions are drawn from the above graph.
i. The solubility of sodium chloride does not vary appreciably as the maximum solubility is achieved at normal temperature. In fact, there is only 10 % increase in solubility between 0 Ëš to 100 ËšC.
ii. The dissolution process of ammonium nitrate is endothermic, the solubility increases steeply with increase in temperature.
iii. In the case of ceric sulphate, the dissolution is exothermic and the solubility decreases with increase in temperature.
iv. Even though the dissolution of calcium chloride is exothermic, the solubility increases moderately with increase in temperature. Here, the entropy factor also plays a significant role in deciding the position of the equilibrium.
Gaseous solute in liquid solvent:
In the case of gaseous solute in liquid solvent, the solubility decreases with increase in temperature. When a gaseous solute dissolves in a liquid solvent, its molecules interact with solvent molecules with weak intermolecular forces. When the temperature increases, the average kinetic energy of the molecules present in the solution also increases. The increase in kinetic energy breaks the weak intermolecular forces between the gaseous solute and liquid solvent which results in the release of the dissolved gas molecules to the gaseous state. Moreover, the dissolution of most of the gases in liquid solvents is an exothermic process, and in such processes, the increase in temperature decreases the dissolution of gaseous molecules.
Activity:
Open the soda bottle and put a balloon over it. The balloon will inflate with the released carbon dioxide from the soda. Carry out the same experiment by placing the soda bottle in a container of hot water. You will observe the balloon is inflated much faster now. This shows the decrease in solubility of gases in solution with increase in temperature. In the rivers where hot water is discharged from industrial plants, the aquatic lives are less sustained due to the decreased availability of dissolved oxygen.
When pressure is increased
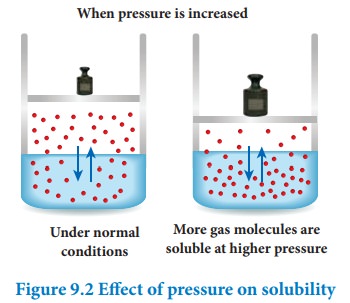
Effect of pressure:
Generally the change in pressure does not have any significant effect in the solubility of solids and liquids as they are not compressible. However, the solubility of gases generally increases with increase of pressure.
Consider a saturated solution of a gaseous solute dissolved in a liquid solvent in a closed container. In such a system, the following equilibrium exists.
Gas (in gaseous state) ⇆ Gas (in solution)
According to Le-Chatelier principle, the increase in pressure will shift the equilibrium in the direction which will reduce the pressure. Therefore, more number of gaseous molecules dissolves in the solvent and the solubility increases.
Related Topics