Chapter: 11th Chemistry : UNIT 9 : Solutions
Determination of molar mass weights from relative lowering of vapour pressure
Relative lowering of vapour pressure (ΔP)
The vapour pressure of a solution containing a nonvolatile, non-electrolyte solute is always lower than the vapour pressure of the pure solvent. Consider a closed system in which a pure solvent is in equilibrium with its vapour. At equilibrium the molar Gibbs free energies of solvent in the liquid and gaseous phase are equal (ΔG = 0). When a solute is added to this solvent, the dissolution takes place and its free energy (G) decreases due to increase in entropy. In order to maintain the equilibrium, the free energy of the vapour phase must also decrease. At a given temperature, the only way to lower the free energy of the vapour is to reduce its pressure. Thus the vapour pressure of the solution must decrease to maintain the equilibrium.
We know that from the Raoult's law the relative lowering of the vapour pressure is equal to the mole fraction of the solute (equation 9.10)
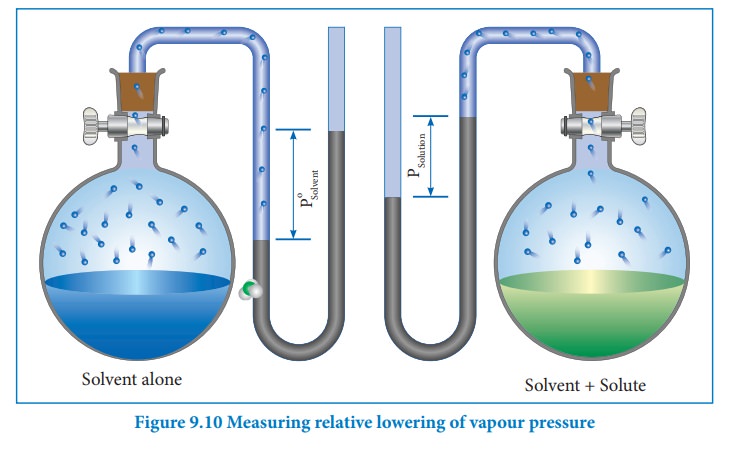
From the above equation,it is clear that the relative lowering of vapour pressure depends only on the mole fraction of the solute (xB) and is independent of its nature. Therefore, relative lowering of vapour pressure is a colligative property.
Determination of molar mass weights from relative lowering of vapour pressure
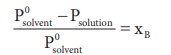
The measurement of relative lowering of vapour pressure can be used to determine the molar mass of a nonvolatile solute. For this purpose, a known mass of the solute is dissolved in a known quantity of solvent. The relative lowering of vapour pressure is measured experimentally.
According to RaoultŌĆÖs law the relative lowering of vapor pressure is,
Let w A and wB be the weights of the solvent and solute respectively and their corresponding molar masses are MA and MB, then the mole fraction of the solute xB is
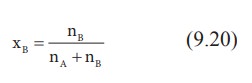
Here, nA & nB are the moles of the solvent and the solute respectively. For dilute solutions nA>>nB. Hence nA +nB Ōēł nA. Now
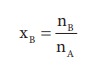
Number of moles of solvent and the solute are,
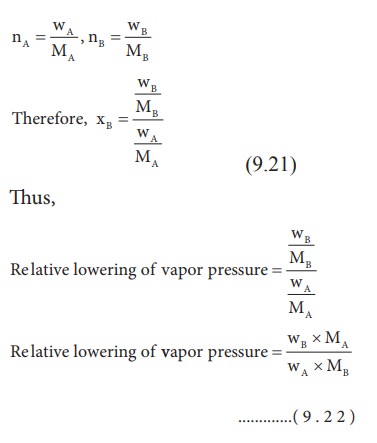
From the equation (9.35) the molar mass of the solute (MB) can be calculated using the known the values of wA, wB, MA and the measured relative lowering of vapour pressure.
Example Problem 3:
An aqueous solution of 2 % nonvolatile solute exerts a pressure of 1.004 bar at the boiling point of the solvent. What is the molar mass of the solute when PA┬░ is 1.013 bar?
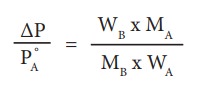
In a 2 % solution weight of the solute is 2 g and solvent is 98 g
╬öP = PA┬░ ŌĆō Psolution= 1.013 -1.004 bar = 0.009 bar
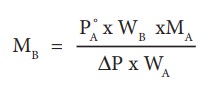
MB = 2 x 18 x 1.013/(98 x 0.009)
= 41.3 g mol-1
Related Topics