Chapter: Genetics and Molecular Biology: Protein Synthesis
Tricking the Translation Machinery into Initiating - Protein Synthesis
Tricking the Translation Machinery into Initiating
Once
messenger has bound to the smaller ribosomal subunit and then a larger subunit
has been added, translation can begin. Escherichia
coli proteins initiate primarily at AUG codons with a peptide chain analog
in which part of the initiating amino acid looks like a peptide bond (Fig.
7.11). This permits the system to form the first real peptide bond with
essentially the same machinery as it uses to form subsequent peptide bonds.
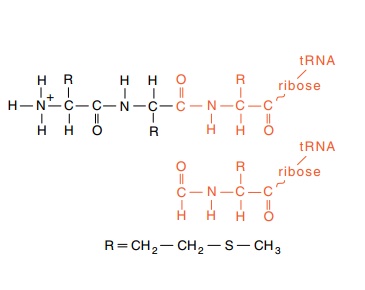
Figure 7.11 The structure of apolypeptide and the similar struc-ture generated by formylation of an amino acid. The initiation amino acid is methionine, whose side group R is shown.
The
initiation analog is N-formyl methionine. Rather than utilize an entirely
separate charging pathway with a separate tRNA synthetase, the cells formylate
a methionine after it has been put on a tRNAMet. This is, however, a
special tRNA called tRNAMetf and it is used only for
initiation.
Methionine bound to the second type of tRNAMet cannot be formylated,
and this is used only for elongation at internal AUG codons. This scheme
prevents the difficulties that would arise from attempting to initiate a
protein with an unformylated methionine or attempting to put a formylated methionine
on the interior of a protein. A new question
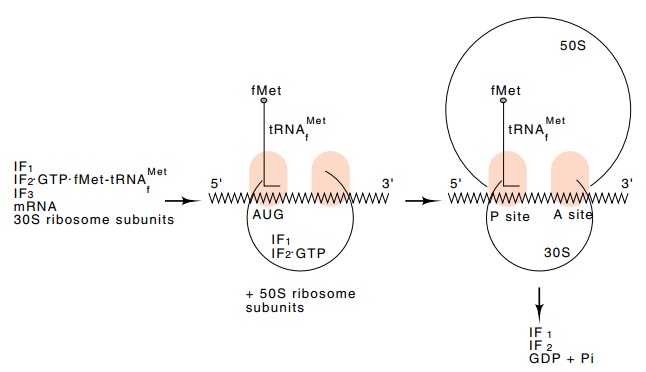
Figure
7.12 Formation of a translation
initiation complex.
The two
Met-tRNAs are distinguished by a set of proteins used in the initiation and
elongation processes. These proteins carry the charged tRNA molecules into the
ribosome (Fig. 7.12). Interactions between the proteins and the
ribosome-messenger complex help hold the tRNA-pro-tein complex in the ribosome.
The most important of these interactions is that between the three-base
anticodon of the tRNA and the three-base codon in the messenger. It is these
codon-anticodon interactions that specify the f-Met initiating amino acid and
successive amino acids along the polypeptide chain. The protein IF2,
initiation factor 2, carries the f−met−tRNAMetf
into the site normally occupied by the growing peptide
chain,
the P site, whereas all other charged tRNAs, including met-tRNA, are carried
into the other site, the acceptor, A, site, by the elongation factors. Thus
N-formyl methionine can be incorporated only at the beginning of a polypeptide.
In
addition to the initiation factor IF2, two other proteins, IF1
and IF3, are also used during the initiation steps. Factor IF1
accelerates the initiation steps but is not absolutely required, and it can be
assayed invitro by its acceleration
of3H-Met-tRNAMetbinding to ribosomes. IF3binds
to the 30S subunit to assist the initiation process.
The initiation of translation in eukaryotes shows some similarity to the process used in bacteria. Two methionine tRNAs are used, one for initiation and one for elongation, but the methionine on the initiating tRNA is not formylated. Methionine on this tRNA, however, can be formylated by the bacterial formylating enzyme. It appears as if the eukaryotic initiation system has evolved from the bacterial system to the point that the formyl group is no longer necessary for protein synthesis, but the tRNAMetf is still similar to its progenitor bacterial tRNAMetf.
Related Topics