Chapter: Genetics and Molecular Biology: Protein Synthesis
Balancing Synthesis of Ribosomal Components - Protein Synthesis
Balancing Synthesis of Ribosomal Components
Although the synthesis of the individual components
of ribosomes may be rather well regulated, a slight imbalance in the synthesis
of one component could eventually lead to elevated and potentially toxic levels
of that component. Synthesis of the ribosomal RNAs in bacteria are kept in
balance by a simple mechanism. As we have already seen, these RNAs are
synthesized in one piece by an RNA polymerase that initiates at a promoter and
transcribes across the genes for the three RNAs. Different mechanisms are used
to maintain balanced synthesis of some of the ribosomal proteins. In one case,
one of the proteins encoded in a ribosomal protein operon reduces translation
of all the proteins in that operon. This effect is called translational
repression.
The finding that a ribosomal protein represses
translation of proteins only from the same operon provides an efficient means
for the cell to maintain balanced synthesis of all the ribosomal proteins.
Suppose that some ribosomal proteins began to accumulate because their
synthesis is a little faster than the other proteins and the rRNA. Then, as the
level of these proteins begins to rise in the cytoplasm, they begin to repress
How do we know about translational repression? The
main clue came from careful measurements on cells with an increased number of
genes coding for some of the ribosomal proteins. The increased copy number
might have been expected to increase the synthesis of the corresponding
proteins, but it did not. The synthesis of the mRNA for these proteins did
increase as expected, and therefore it appeared that extra ribosomal proteins
in the cell inhibited translation of their own mRNA. Proof of the idea of
translational repression came from in
vitro studies in which levels of individual free ribosomal proteins could
be adjusted at will. The addition of DNA containing genes for some of the
ribosomal proteins and properly prepared cell extract permits transcription and
translation to yield ribosomal proteins synthesized in vitro. Nomura found that addition of the appropriate free
ribosomal proteins to such a system repressed synthesis of the proteins encoded
by the same operon as the added protein.
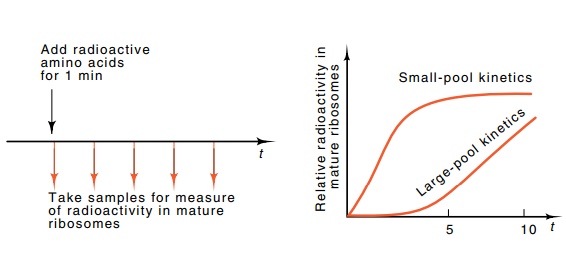
Figure 7.28 Determination of the pool size of ribosomal protein by the kineticsof a pulse of radioactive amino acids into ribosomal protein.
Not surprisingly, a ribosomal protein that
regulates synthesis of a group of proteins binds to the mRNA to effect the
repression. The structure of the binding region on the mRNA for some of these
proteins is the same as the structure the protein binds to in the rRNA in the
ribosome.
Global limits can be placed on the accuracy with
which the synthesis of ribosomal components is balanced. A short pulse of
radioactive amino acid is provided to the cells, and the total pool of all
ribosomal proteins can be determined by measuring the kinetics of incorporation
of label into mature ribosomes (Fig. 7.28). The results show that the pool
contains less than a five-minute supply of ribosomal proteins. Similarly, the
pool size of each individual ribosomal protein can be measured. The results of
these experiments show that most of the ribosomal proteins also have very small
intracellular pools.
We have seen that the mechanisms regulating
ribosome synthesis have good reason to be sophisticated, and, indeed those
aspects that have been investigated have turned out to be complicated. Much of
the biochemistry and perhaps even much of the physiology of ribosome regulation
remain to be worked out. In bacteria and other single-celled organisms such as
yeast, it is likely that most of the regulation mecha - nism can be dissected
by a combination of physiology, genetics, and biochemistry. It will be
interesting to see if analogous problems in higher organisms can also be solved
without the availability of genetics.
Related Topics