Chapter: Genetics and Molecular Biology: Protein Synthesis
Translocation - Protein Synthesis
Translocation
Following formation of a peptide bond, the P site
of the ribosome contains an uncharged tRNA, and the A site contains a tRNA
linked to the growing peptide chain. Translocation is the process of recocking
the elongation mechanism. The uncharged tRNA in the P site is moved to the
exit, or E site, messenger translocates three bases toward the P site,
The translocation process itself requires hydrolysis of a GTP
molecule that has been carried to the ribosome by the EF-G or G factor. Since
the elongation factors are used once for each amino acid added, a large number
of molecules of each must be present in the cell to support protein synthesis.
It is also logical that their level should parallel the level of ribosomes,
and, indeed, as growth rate varies, their levels do keep pace with the levels
of ribosomes. With the entry of a charged tRNA into the A site of the ribosome,
the uncharged tRNA in the E site is released.
At some time during the growth of the peptide
chains, the N-terminal amino acid is modified. Approximately 40% of the
proteins isolated from E. coli are found to begin with methionine,
but since all initiate with N-formyl methionine, the remaining 60% must lose at
least the N-terminal methionine. Similarly, the 40% of the proteins that do
begin with methionine all lack the formyl group. Thus the formyl group must be
removed after protein synthesis has initiated. Examination of nas-cent
polypeptide chains on ribosomes shows that the formyl group is missing if they
are larger than about 30 amino acids, and therefore the
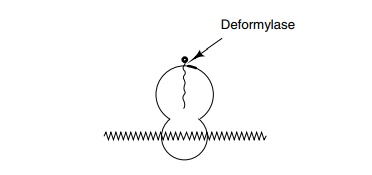
The deformylase is a very labile enzyme that is
exceedingly sensitive to sulfhydryl reagents. Since many other enzymes isolated
from the same cells require the same sulfhydryl reagents for stability, it may
be that the deformylase is normally bound to some structure that contrib-utes
to its stability, and when it is isolated from extracts and partially purified,
it is particularly labile in its unnatural environment.
Related Topics