Chapter: 11th Physics : UNIT 9 : Kinetic Theory of Gases
Summary - Physics: Kinetic Theory of Gases
SUMMARY
Kinetic theory explains the microscopic origin of macroscopic
parameters like temperature, pressure.
The pressure exerted on the walls of gas container is due to the
momentum imparted by the gas molecules on
the walls.
The pressure 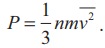 . The pressure is directly proportional to the number density, mass
of molecule and mean square speed.
. The pressure is directly proportional to the number density, mass
of molecule and mean square speed.
The temperature of a gas is a measure of the average translational
kinetic energy per molecule of the gas. The
average kinetic energy per molecule is directly proportional to absolute
temperature of gas and independent of nature of molecules.
The pressure is also equal to 2/3 of internal energy per unit
volume.
The rms speed of gas molecules = v = 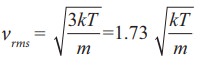
The
average speed of gas molecules 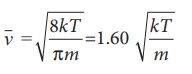
The
most probable speed of gas molecules vmp
= 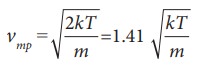
Among
the speeds vrms is the
largest and vmp is the
least
vrms > v >vmp
The number of gas molecules in the range of speed v to v+dv is
given by Maxwell-Boltzmann distribution
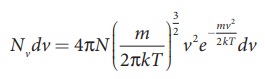
The minimum number of independent coordinates needed to specify
the position and configuration of a
thermodynamical system in space is called the degrees of freedom of the system.
If a sample of gas has N molecules, then the total degrees of freedom f = 3N.
If there are q number of constraints then total degrees of freedom f = 3N-q.
For a monoatomic molecule, f = 3
For
a diatomic molecule (at normal temperature), f = 5
For a diatomic molecule (at high temperature), f = 7
For a diatomic molecule (at high temperature), f = 7
For
a triatomic molecule (linear type), f = 7
For
a triatomic molecule (non-linear type), f = 6
The
average kinetic energy of sample of gas is equally distributed to all the
degrees of freedom. It is called law of equipartition of energy. Each degree of
freedom will get 1/2 kT energy.
The
ratio of molar specific heat at constant pressure and constant volume of a gas
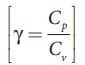
For
Monoatomic
molecule: 1.67
Diatomic
molecule (Normal temperature) : 1.40
Diatomic
molecule (High temperature): 1.28
Triatomic
molecule (Linear type): 1.28.
Triatomic
molecule (Non-linear type): 1.33
The mean free path λ = kT / ( √ 2πd2p) . The mean free path is directly proportional to temperature and inversely proportional
to size of the molecule and pressure of the molecule
The
Brownian motion explained by Albert Einstein is based on kinetic theory. It
proves the reality of atoms and molecules.
Related Topics