Kinetic Theory of Gases | Physics - Short Questions and Answer | 11th Physics : UNIT 9 : Kinetic Theory of Gases
Chapter: 11th Physics : UNIT 9 : Kinetic Theory of Gases
Short Questions and Answer
Kinetic Theory of Gases (Physics)
Short answer questions
1. What is the microscopic origin of pressure?
With the help of kinetic theory of gases, the pressure is linked to the velocity of molecules,
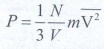
N
- Avogadro Number;
V
- Volume;
![]() - Avogadro velocity molecules,
- Avogadro velocity molecules,
m - mass of a molecule.
2. What is the microscopic origin of temperature?
Average
Kinetic Energy / Molecule :
KE = ε = 3/2 NkT
3. Why moon has no atmosphere?
The escape speed of gases on the surfaces of Moon is much less than the root mean square speeds of gases due to low gravity. Due to this all the gases escape from the surface of the Moon.
4. Write the expression for rms speed, average speed and most probable speed of a gas molecule.
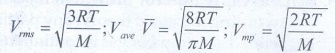
5. What is the relation between the average kinetic energy and pressure?
The
internal energy of the gas is given by
U = 3/2 NkT
The
above equation can also be written as U = 3/2 PV, Since PV = NkT
P = 2/3 U/V
= 2/3 u …………(1)
From
the equation (1), we can state that the pressure of the gas is equal to two
thirds of internal energy per unit volume or internal energy density, u =
U / V
Pressure
in terms of mean kinetic energy density using equation.
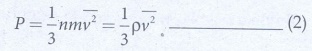
where
ρ = nm = mass density (n is number density)
Multiply
and divide R.H.S of equation (2) by 2, we get
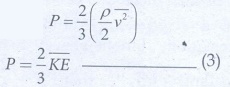
From
the equation (3), pressure is equal to 2/3 of mean kinetic energy per unit
volume.
6. Define the term degrees of freedom.
The
minimum number of independent coordinates needed to specify the position and
configuration of a thermo-dynamical system in space.
7. State the law of equipartition of energy.
According
to kinetic theory, the average kinetic energy of system of molecules in thermal
equilibrium at temperature T is uniformly distributed to all degrees of freedom
will get ½ kT of energy. This is called law of equipartition of energy.
8. Define mean free path and write down its expression.
Average
distance travelled by the molecule between collisions is called mean free path
(λ). The mean free path based on kinetic theory.
9. Deduce Charles’ law based on kinetic theory.
Charles'
law:
From the equation
P = 2/3 (U/V
) = 2/3(u), we
get PV = 2/3 (U)
For
a fixed pressure, the volume of the gas is proportional to internal energy of
the gas or average kinetic energy of the gas and the average kinetic energy is
directly proportional to absolute temperature. It implies that V ∝ T or V / T =
constant
10. Deduce Boyle’s law based on kinetic theory.
Boyle's
law:
From equation P = 2/3 (U/V) = 2/3 (u),
we
know that PV = 2/3 U .
But
the internal energy of an ideal gas is equal to N times the average kinetic
energy (∈) of each molecule. U = N ∈
For
a fixed temperature, the average translational kinetic energy ∈ will remain constant. It implies
that PV = 2/3 N∈ . Thus
PV = constant
Therefore,
pressure of a given gas is inversely proportional to its volume provided the
temperature remains constant. This is Boyle's law.
11. Deduce Avogadro’s law based on kinetic theory.
This
law states that at constant temperature and pressure, equal volumes of all
gases contain the same number of molecules. For two different gases at the same
temperature and pressure, according to kinetic theory of gases,
From
equation
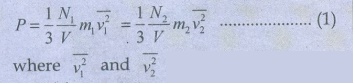
are the mean square speed for two gases and N1
and N2 are the number of gas molecules in two different gases. At
the same temperature, average kinetic energy per molecule is the same for two
gases.
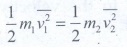 ………….(2)
………….(2)
Dividing
the equation 1 by 2 we get N1 = N2
This
is Avogadro's law. It is sometimes referred to as Avogadro's hypothesis or
Avogadro's Principle.
12. List the factors affecting the mean free path.
●
Mean free path increases with increasing temperature. As the temperature
increases, the average speed of each molecule will increase. It is the reason
why the smell of hot sizzling food reaches several meter away than smell of
cold food.
●
Mean free path increases with decreasing pressure of the gas and diameter of
the gas molecules.
13. What is the reason for Brownian motion?
According to kinetic theory, any particle suspended in a liquid or gas is continuously bombarded from all the directions so that the mean free path is almost negligible. This leads to the motion of the particles in a random and zig-zag manner.
Related Topics