Kinetic Theory of Gases - Most probable speed | 11th Physics : UNIT 9 : Kinetic Theory of Gases
Chapter: 11th Physics : UNIT 9 : Kinetic Theory of Gases
Most probable speed
Most
probable speed (Vmp)
It
is defined as the speed acquired by most of the molecules of the gas.
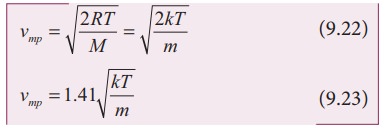
The
derivation of equations (9.20), (9.22) is beyond the scope of the book
Comparison of vrms, v
and vmp
Among
the speeds vrms is the
largest and vmp is the
least
vrms > v > vmp
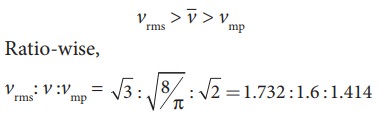
EXAMPLE 9.3
Ten
particles are moving at the speed of 2, 3, 4, 5, 5, 5, 6, 6, 7 and 9 m s-1.
Calculate rms speed, average speed and most probable speed.
Solution
The
average speed
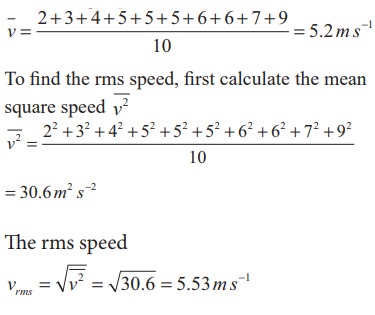
The
most probable speed is 5 ms-1 because three of the particles have
that speed.
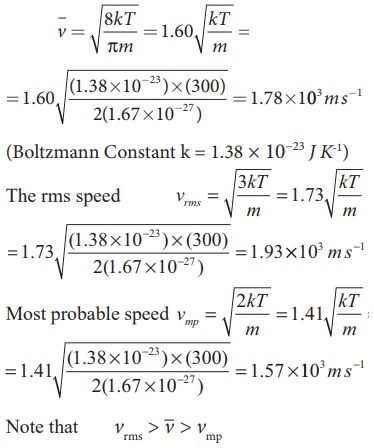
EXAMPLE 9.4
Calculate
the rms speed, average speed and the most probable speed of 1 mole of hydrogen
molecules at 300 K. Neglect the mass of electron.
Solution
The
hydrogen atom has one proton and one electron. The mass of electron is
negligible compared to the mass of proton.
Mass
of one proton = 1.67 × 10−27kg.
One
hydrogen molecule = 2 hydrogen atoms = 2 × 1.67 × 10−27kg.
The
average speed
Related Topics