Kinetic Theory of Gases | Physics - Book Back Numerical Problems | 11th Physics : UNIT 9 : Kinetic Theory of Gases
Chapter: 11th Physics : UNIT 9 : Kinetic Theory of Gases
Book Back Numerical Problems
Kinetic Theory of Gases (Physics)
Numerical Problems
1. A fresh air is composed of nitrogen N2(78%) and oxygen O2(21%). Find the rms speed of N2 and O2 at 20°C.
Solution:
For Nitrogen,
Molar mass m = 0.0280 kg/mol
Temperature T = 20°C = 20 + 273 = 293K
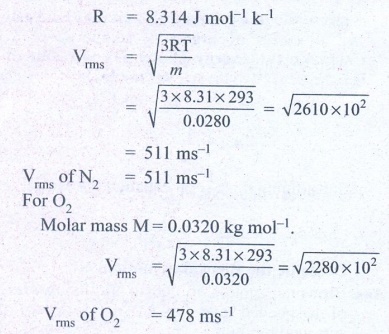
Ans: For vrms = 511 m s-1
For O2vrms = 478 m s-1
2. If the rms speed of methane gas in the Jupiter’s atmosphere is 471.8 m s-1, show that the surface temperature of Jupiter is sub-zero.
Solution:
RMS speed of methane gas = 471.8 ms-1 =Vrms
Sub-zero temperature Molar mass of methane gas
= 16.04 × 10-3 kg mol-1
Surface temperature of Jupiter T = ?
Gas constant R =8.31
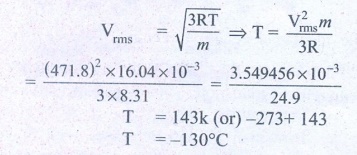
Ans: -130°C
3. Calculate the temperature at which the rms velocity of a gas triples its value at S.T.P.
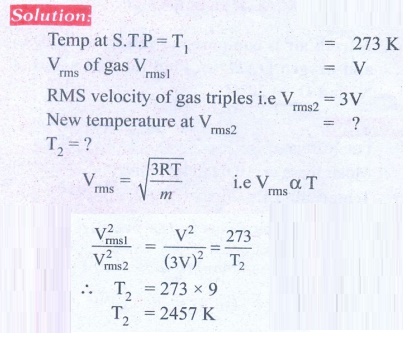
Ans: T1 = 273 K, T2 = 2457 K
4. A gas is at temperature 80°C and pressure 5 × 10-10N m-2. What is the number of molecules per m3 if Boltzmann’s constant is 1.38 × 10-23 J K-1
Solution:
Temperature of gas T = 80°C = 80 + 273 = 353K
Pressure of gas P = 5 × 10-10 Nm-2
Boltzmann’s constant k = 1.38 × 10-23 Jk-1
Volume of gas V = 1 m3
No. of molecules n = ?
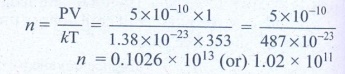
Ans: 1.02 × 1011
5. From kinetic theory of gases, show that Moon cannot have an atmosphere (Assume k = 1.38 × 10-23 J K-1 Temperature T=0°C=273K).
Ans: vescape = vrms= 1.86 km s-1
6. If 1020 oxygen molecules per second strike 4 cm2 of wall at an angle of 30° with the normal when moving at a speed of 2 × 103 m s-1, find the pressure exerted on the wall. (mass of 1 atom = 1.67 × 10-27 kg)
Solution
Mass of 1 O2 atom = 2.67 × 10-26 kg
Mass of 1020 O2 atom = 2.67 × 10-26 kg
= 26.72 × 10-27 × 1020
Momentum P = mv
= 26.72 × 10-7 × 8 × 2 × 103
= 427.5 × 10-4 kgm s-1
Component of momentum normal to wall is 30°
= 427.5 ×10-4 × cos 30° .

Ans: 92.4 N m-2
7. During an adiabatic process, the pressure of a mixture of monatomic and diatomic gases is found to be proportional to the cube of the temperature. Find the value of γ = (Cp/CV)
Solution
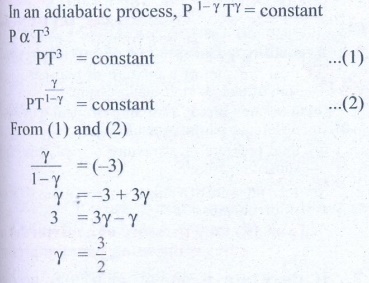
Ans: 3/2
8. Calculate the mean free path of air molecules at STP. The diameter of N2 and O2 is about 3 × 10-10 m
Solution

Ans: λ≈9 × 10-8 m
9. A gas made of a mixture of 2 moles of oxygen and 4 moles of argon at temperature T. Calculate the energy of the gas in terms of RT. Neglect the vibrational modes.
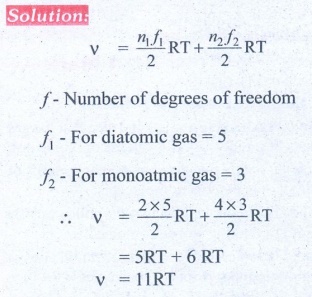
Ans: 11RT
10. Estimate the total number of air molecules in a room of capacity 25 m3 at a temperature of 27°C.
Solution:
Volume of the room V = 25.0m3
Temperature of the room T = 27°C = 300K
Pressure in the room P = estimate
= 1 × 1.013 × 105 Pa
The ideal gas equation relating pressure (P), volume (v) absolute temperature (T) can be written is
PV = KB NT
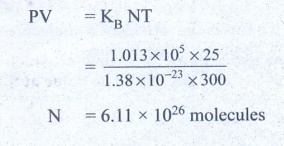
Ans: 6.1 × 1026 molecules
Related Topics