Kinetic Theory of Gases | Physics - Degrees of Freedom | 11th Physics : UNIT 9 : Kinetic Theory of Gases
Chapter: 11th Physics : UNIT 9 : Kinetic Theory of Gases
Degrees of Freedom
DEGREES
OF FREEDOM
Definition
The minimum number of independent coordinates needed to specify the position and configuration of a thermo-dynamical system in space is called the degree of freedom of the system.
Example:
1.
A free particle moving along x-axis needs only one coordinate to specify it
completely. So its degree of freedom is one.
2.
Similarly a particle moving over a plane has two degrees of freedom.
3.
A particle moving in space has three degrees of freedom.
Suppose
if we have N number of gas molecules in the container, then the total number of
degrees of freedom is f = 3N.
But,
if the system has q number of
constraints (restrictions in motion) then the degrees of freedom decreases and
it is equal to f = 3N-q where N is
the number of particles.
1. Monoatomic molecule
A
monoatomic molecule by virtue of its nature has only three translational
degrees of freedom.
Therefore
f = 3
Example: Helium, Neon, Argon
2. Diatomic molecule
There
are two cases.
1. At Normal temperature
A
molecule of a diatomic gas consists of two atoms bound to each other by a force
of attraction. Physically the molecule can be regarded as a system of two point
masses fixed at the ends of a massless elastic spring.
The center of mass lies in the center of the diatomic molecule. So, the motion of the center of mass requires three translational degrees of freedom (figure 9.5 a). In addition, the diatomic molecule can rotate about three mutually perpendicular axes (figure 9.5 b).
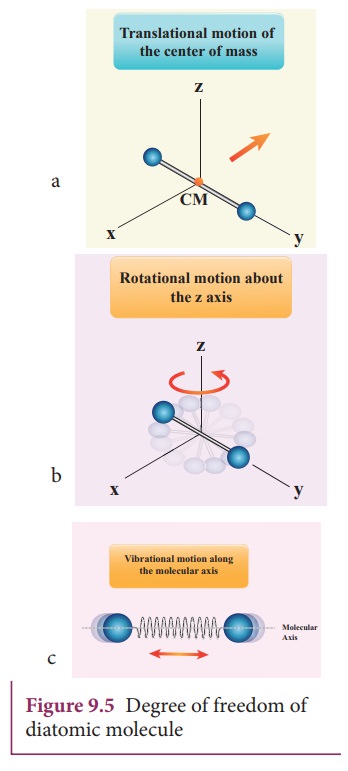
But the moment of inertia
about its own axis of rotation is negligible (about y axis in the figure 9.5).
Therefore, it has only two rotational degrees of freedom (one rotation is about
Z axis and another rotation is about Y axis). Therefore totally there are five
degrees of freedom.
f = 5
2. At High Temperature
At
a very high temperature such as 5000 K, the diatomic molecules possess
additional two degrees of freedom due to vibrational motion[one due to kinetic
energy of vibration and the other is due to potential energy] (Figure 9.5c). So
totally there are seven degrees of freedom.
f = 7
Examples: Hydrogen, Nitrogen, Oxygen.
3. Triatomic molecules
There
are two cases.
Linear triatomic molecule
In
this type, two atoms lie on either side of the central atom as shown in the
Figure 9.6
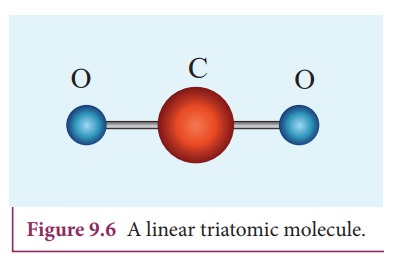
Linear
triatomic molecule has three translational degrees of freedom. It has two
rotational degrees of freedom because it is similar to diatomic molecule except
there is an additional atom at the center. At normal temperature, linear
triatomic molecule will have five degrees of freedom. At high temperature it
has two additional vibrational degrees of freedom.
So
a linear triatomic molecule has seven degrees of freedom.
Example: Carbon dioxide.
Non-linear triatomic molecule
![]()
![]() In this case, the three atoms lie at the vertices of a
triangle as shown in the Figure 9.7
In this case, the three atoms lie at the vertices of a
triangle as shown in the Figure 9.7
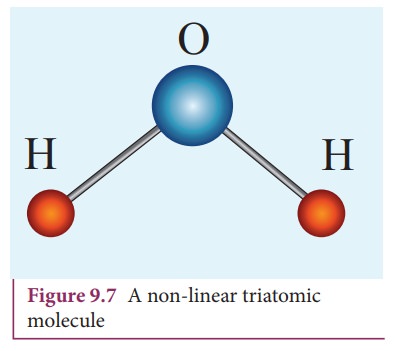
It
has three translational degrees of freedom and three rotational degrees of
freedom about three mutually orthogonal axes. The total degrees of freedom, f =
6
Example: Water, Sulphurdioxide.
Related Topics