Chapter: 11th Physics : UNIT 9 : Kinetic Theory of Gases
Kinetic interpretation of temperature
Kinetic
interpretation of temperature
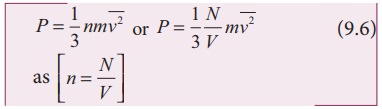
To understand the microscopic origin of temperature in the same way,
Rewrite
the equation (9.6)
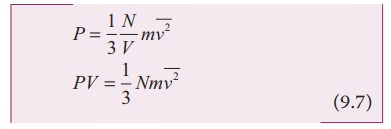
Comparing
the equation (9.7) with ideal gas equation PV=NkT,
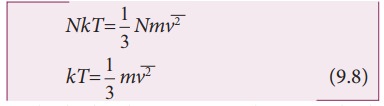
Multiply
the above equation by 3/2 on both sides,

R.H.S
of the equation (9.9) is called average kinetic energy of a single molecule ![]() .
.
The
average kinetic energy per molecule

Equation
(9.9 ) implies that the temperature of a gas is a measure of the average
translational kinetic energy per molecule of the gas.
Equation 9.10 is a very important result from kinetic theory of gas. We can infer the following from this equation.
(i) The average kinetic energy of the molecule is directly
proportional to absolute temperature of the gas. The equation (9.9) gives the
connection between the macroscopic world (temperature) to microscopic world
(motion of molecules).
(ii)
The average kinetic energy of each molecule depends only on temperature of the
gas not on mass of the molecule. In other words, if the temperature of an ideal
gas is measured using thermometer, the average kinetic energy of each molecule
can be calculated without seeing the molecule through naked eye.
By
multiplying the total number of gas molecules with average kinetic energy of
each molecule, the internal energy of the gas is obtained.
Internal
energy of ideal gas U =

From
equation (9.11), we understand that the internal energy of an ideal gas depends
only on absolute temperature and is independent of pressure and volume.
EXAMPLE 9.1
A
football at 27°C has 0.5 mole of air molecules. Calculate the internal energy
of air in the ball.
Solution
The
internal energy of ideal gas = 3/2 NkT.
The number of air molecules is given in terms of number of moles so, rewrite
the expression as follows
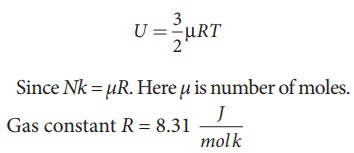
Temperature
T =273+27=300K
U
= 3/2 × 0.5 × 8.31 × 300 = 1869.75J
This
is approximately equivalent to the kinetic energy of a man of 57 kg running
with a speed of 8 m s-1.
Related Topics