Chapter: 11th Physics : UNIT 9 : Kinetic Theory of Gases
Brownian Motion
BROWNIAN
MOTION
In
1827, Robert Brown, a botanist reported that grains of pollen suspended in a
liquid moves randomly from one place to other. The random (Zig - Zag path)
motion of pollen suspended in a liquid is called Brownian motion. In fact we
can observe the dust particle in water moving in random directions. This discovery
puzzled scientists for long time. There were a lot of explanations for pollen
or dust to move in random directions but none of these explanations were found
adequate. After a systematic study, Wiener and Gouy proposed that Brownian
motion is due to the bombardment of suspended particles by molecules of the
surrounding fluid. But during 19th century people did not accept that every
matter is made up of small atoms or molecules. In the year 1905, Einstein gave
systematic theory of Brownian motion based on kinetic theory and he deduced the
average size of molecules.
![]()
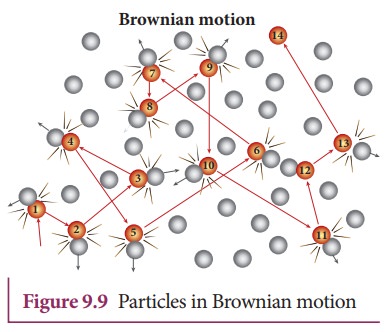
![]() According to kinetic theory, any particle suspended in a
liquid or gas is continuously bombarded from all the directions so that the
mean free path is almost negligible. This leads to the motion of the particles
in a random and zig–zag manner as shown in Figure 9.9. But when we put our hand
in water it causes no random motion because the mass of our hand is so large
that the momentum transferred by the molecular collision is not enough to move
our hand.
According to kinetic theory, any particle suspended in a
liquid or gas is continuously bombarded from all the directions so that the
mean free path is almost negligible. This leads to the motion of the particles
in a random and zig–zag manner as shown in Figure 9.9. But when we put our hand
in water it causes no random motion because the mass of our hand is so large
that the momentum transferred by the molecular collision is not enough to move
our hand.
Factors affecting Brownian Motion
1.
Brownian motion increases with increasing temperature.
2.
Brownian motion decreases with bigger particle size, high viscosity and density
of the liquid (or) gas.
Related Topics